Formulation and Delivery - Chemical
Category: Poster Abstract
(M1030-11-73) Manufacturing and Elucidating the Drug Release Mechanisms of Long-Acting Ethylene Vinyl Acetate-Based Implants

Angela Ren, BS (she/her/hers)
Graduate Student
University of Texas At Austin
Austin, Texas, United States
Angela Ren, BS (she/her/hers)
Graduate Student
University of Texas At Austin
Austin, Texas, United States
Ziyue Zhong, MS (he/him/his)
Research assistant
University of Texas at Austin
Austin, Texas, United States- YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - WS
William Smith, Ph.D.
US Food and Drug Administration
Silver Springs, Maryland, United States - BQ
Bin Qin, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - TL
Tony Listro, MS
Sever Pharma Solutions
Putnam, Connecticut, United States - FZ
Feng Zhang, Ph.D. (he/him/his)
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The advantages of ethylene vinyl acetate (EVA)-based long-acting implants for drug delivery include improved patient compliance, efficacy, and safety [1]. These complex dosage forms contain dispersed and dissolved drug in a polymeric core coupled with a rate-controlling skin layer. Drug must first dissolve in order to release by diffusion. Release is complex due to presence of dispersed and dissolved drug and a skin layer on the implant length but not the ends. The purpose of this study is to elucidate the release mechanisms of long-acting, EVA-based dispersed-drug implants to support rational design of future polymeric implant systems based on transport properties and development of regulatory guidance for generic drugs.
Methods: Implants were manufactured using a coextrusion process consisting of an 18 mm twin-screw (Leistritz) and single-screw extruder (Davis-Standard) connected to a die head, water bath, laser micrometer, and puller (Figure 1(a)). The core comprised 30% etonogestrel loaded in EVA 28 and skin was EVA 15 (70 μm thickness). Each implant was 2.1 mm in diameter, 4 cm long, and contained 38 mg drug. Drug release was measured using an incubator shaker at 37ºC and 150 rpm. Release media was 0.00075% Tween 80 in water. Whole implant (skin and ends) release was evaluated using 4 cm long segments. Core, skin or ends only release was measured using 2 cm long segments with desired sections sealed with Loctite 4011 glue, which is impermeable to etonogestrel. Thin sections were prepared using a cryostat (Thermo Scientific) and visualized using polarized light microscopy (Leica). Implants were frozen in liquid nitrogen prior to cutting sections for scanning electron microscopy (FEI).
Results: The whole implant and cross sections are shown in Figure 1. Birefringence indicates crystalline drug embedded in semicrystalline EVA 28 (Figure 1(c)). A magnified view suggests intimate mixing between drug and polymer (Figure 1(d)). Drug is dispersed and dissolved in EVA 28. During extrusion, a portion of etonogestrel is solubilized in EVA 28 at elevated temperatures. Solubilized drug recrystallizes during storage but remains supersaturated. The initial burst release through skin is due to (1) supersaturation in both skin and core and (2) release of ½ of drug dissolved in the skin. Following the initial burst, the dispersed drug reservoir theoretically maintains constant dissolved concentration in the core, resulting in steady-state release through skin [1]. Based on EVA 15 etonogestrel transport properties determined from diffusion experiments, the steady-state release rate is 38 μg/day. Actual release through skin is dependent on the ratio of dissolution rate/diffusion rate (Figure 2). The actual rate deviates slightly from predicted (Figure 3(a)) due to the formation of a depletion layer because dissolved drug cannot distribute uniformly in the core and a decrease in the ratio of dissolution rate/diffusion rate as drug is gradually depleted from the core. Multiple studies have reported deviations from the t1/2 model for similar systems, prompting our group to investigate the underlying mechanisms of our implant [2, 3]. The initial release period is linear with t and not t1/2, indicating dissolution limited behavior [3, 4]. Predicted Higuchi release from ends was slower than actual release (Figure 3(b)). We hypothesize the discrepancy is due to solution-filled pores being generated as dispersed drug dissolves. The Higuchi model predicts release based on polymer transport properties, but the apparent release is also dependent on media transport properties for systems containing significant porosity due to high drug load. Although solubility is 600-fold lower in release media than in EVA 28 (7.4 μg/mL vs 4500 μg/mL), diffusivity is 104-fold higher (10-5 cm2/s vs 10-9 cm2/s) leading to higher apparent permeability of etonogestrel in EVA 28. The surfaces actively involved in dissolution are restricted to implant ends, so local porosity is significant. Even though the ends make up 2.5% of total surface area, they account for 18% of total release over the first 4 months. Whole implant release was tested after 3 weeks and 6 months of room temperature storage, and the burst was reduced indicating drug recrystallization in the core during storage (Figure 3(c)).
Conclusion: Release from dispersed-drug EVA-based implants is governed by the ratio of drug dissolution rate/diffusion rate in EVA. When drug cannot dissolve in EVA rapidly enough to maintain the saturation at the core-skin interface, release becomes dissolution limited. Skin release deviates from steady-state due formation of a depletion layer which increases diffusion path. Ends release deviates from Higuchi model due to dissolution limited release and increased apparent diffusivity of solution-filled pores. Initial burst is reduced with storage as supersaturated drug recrystallizes in the implant core.
References: 1. Van Laarhoven, J.A.H., Physical-chemical aspects of a coaxial sustained release device based on poly-eva. 2005.
2. Chandrasekaran, S.K. and D.R. Paul, Dissolution-controlled transport from dispersed matrixes. J Pharm Sci, 1982. 71(12): p. 1399-402.
3. Haleblian, J., et al., Steroid release from silicone elastomer containing excess drug in suspension. J Pharm Sci, 1971. 60(4): p. 541-5.
4. Lindstrom, F.T. and J.W. Ayres, Diffusion model for drug release from suspensions II: release to a perfect sink. J Pharm Sci, 1977. 66(5): p. 662-8.
Acknowledgements: This work was supported by the Broad Agency Announcement (BAA) Contract # 75F40122C00019 from the U.S. Food and Drug Administration (FDA). The content reflects the views of the authors and should not be construed to present FDA’s views or policies. The authors wish to express their gratitude to Celanese for providing EVA for this study, and for their valuable insight related to EVA characterization and material properties.
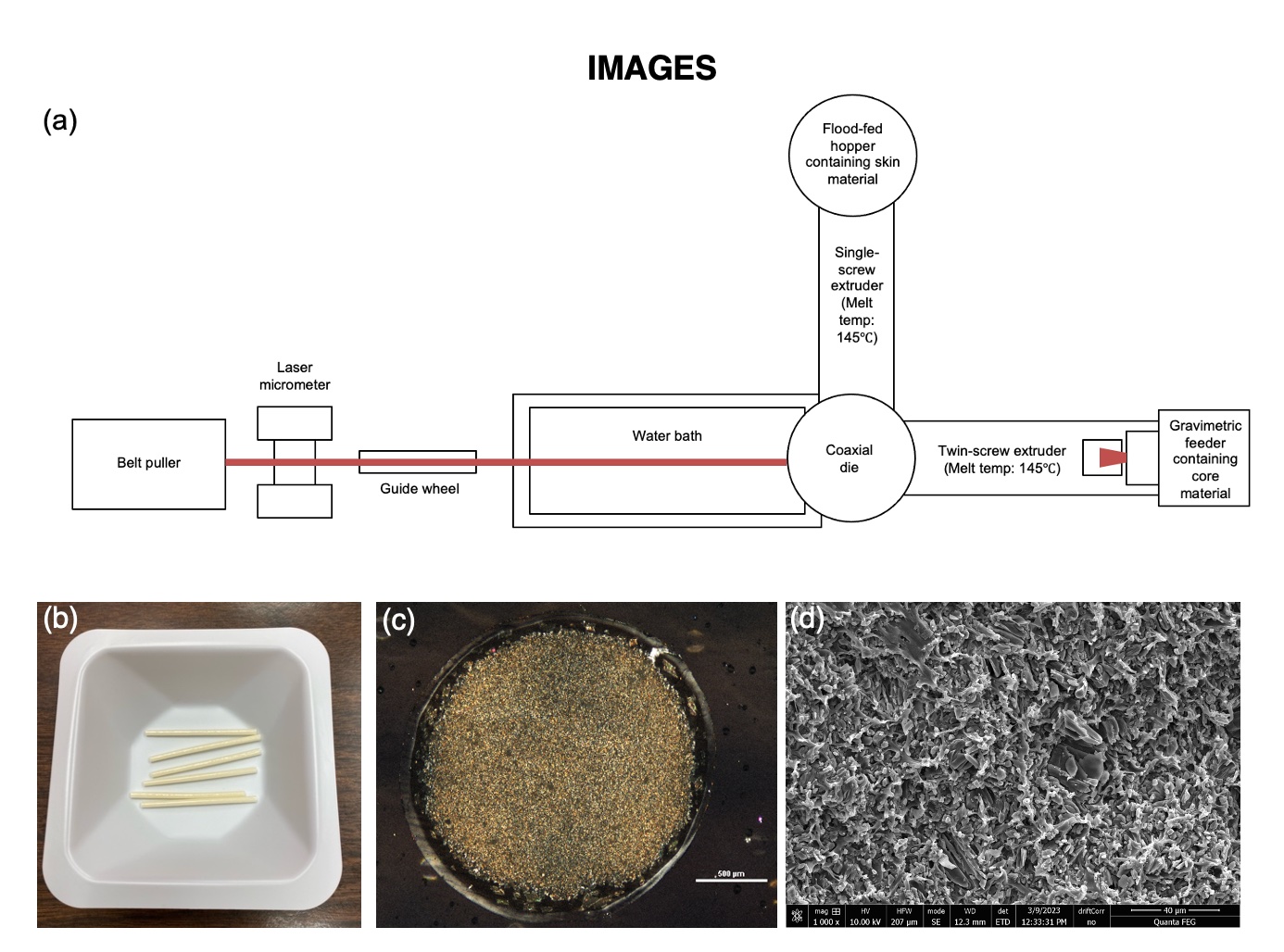 Figure 1. (a) Implant manufacturing process and (b) visualization of whole implants; (c) PLM image of cross section using cross-polarizers and (d) SEM image of dispersed-drug core.
Figure 1. (a) Implant manufacturing process and (b) visualization of whole implants; (c) PLM image of cross section using cross-polarizers and (d) SEM image of dispersed-drug core. 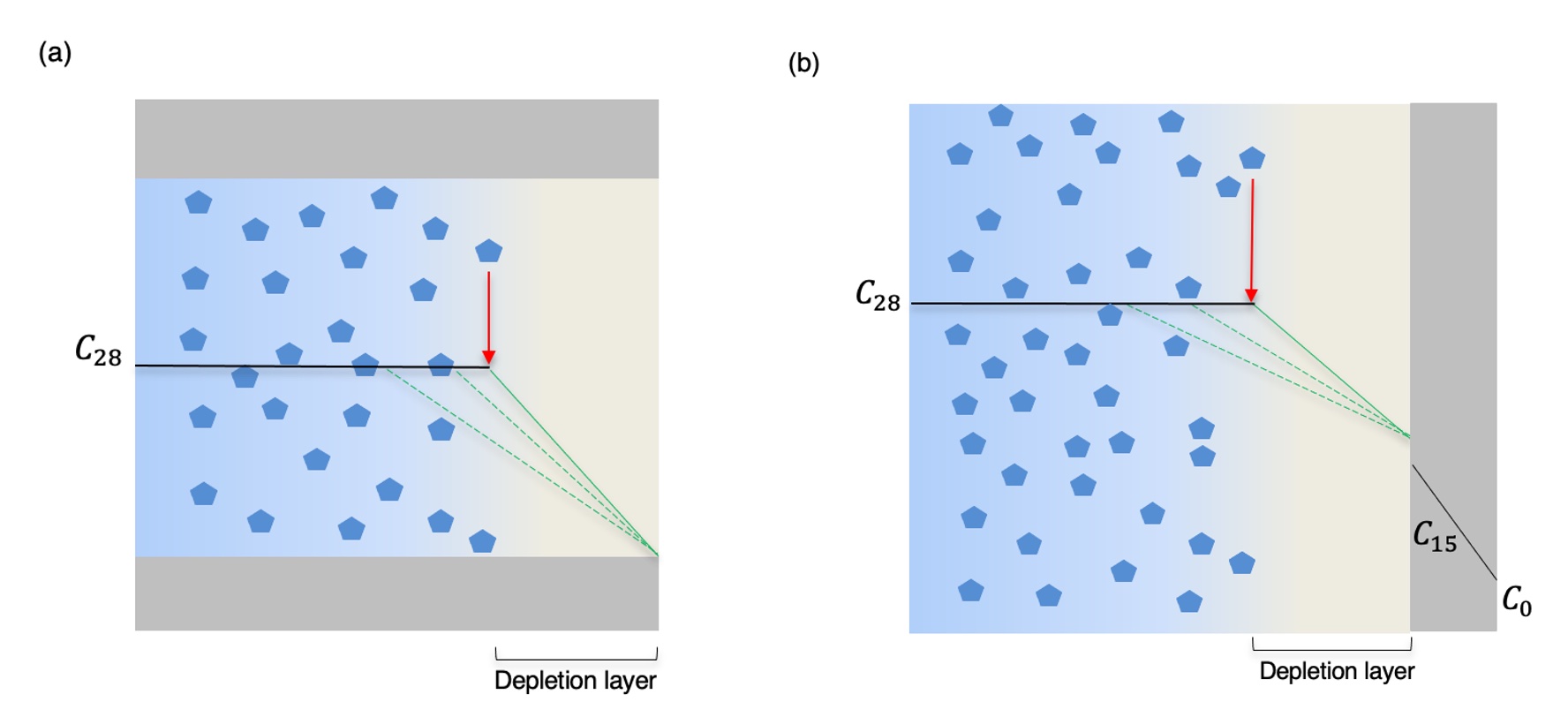 Figure 2. Representative illustration of drug release mechanism through (a) ends and (b) skin. Dark blue indicates dispersed drug, light blue indicates dissolved drug. Red arrows indicate drug dissolution, green lines indicate drug diffusion.
Figure 2. Representative illustration of drug release mechanism through (a) ends and (b) skin. Dark blue indicates dispersed drug, light blue indicates dissolved drug. Red arrows indicate drug dissolution, green lines indicate drug diffusion.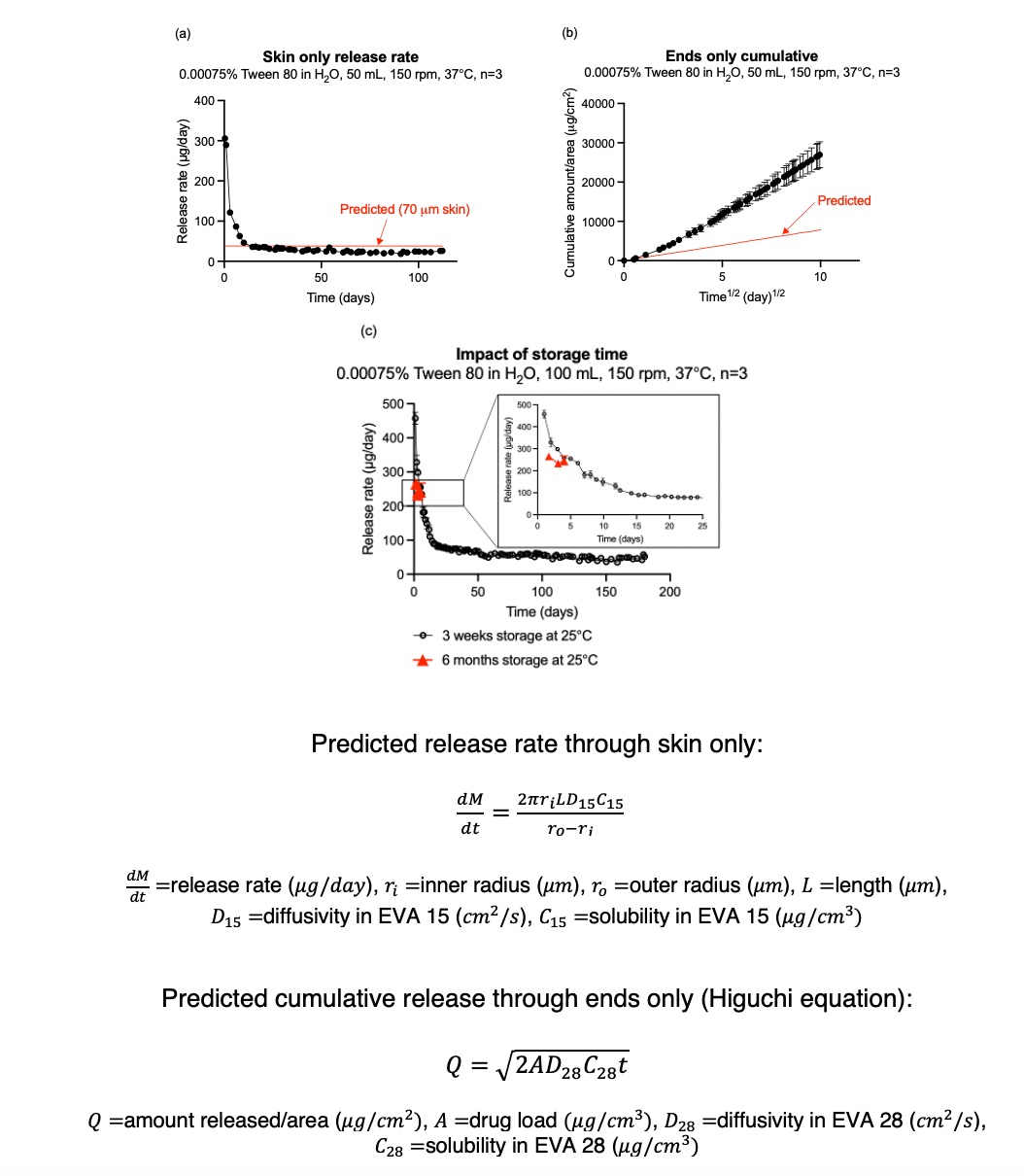 Figure 3. Summary of implant release behavior (a) skin only release rate (2 cm) (b) ends only cumulative release (2 cm) (c) whole implant (skin and ends) release rate (4 cm). The predicted release rate through skin only was calculated using the cylindrical form of Fick’s law. The predicted cumulative release through ends only was calculated using the Higuchi equation.
Figure 3. Summary of implant release behavior (a) skin only release rate (2 cm) (b) ends only cumulative release (2 cm) (c) whole implant (skin and ends) release rate (4 cm). The predicted release rate through skin only was calculated using the cylindrical form of Fick’s law. The predicted cumulative release through ends only was calculated using the Higuchi equation.