Preclinical and Translational Sciences - Chemical
Category: Poster Abstract
(M1430-10-68) Physiologically-Based Pharmacokinetic (PBPK) Modeling of Oral Absorption of 5-flucytosine to Support Development of a Sustained-Release Formulation for Treatment of Cryptococcal Meningoencephalitis
Monday, October 23, 2023
2:30 PM - 3:30 PM ET
- ES
Erik Sjögren, Ph.D. (he/him/his)
Pharmetheus AB
Uppsala, Uppsala Lan, Sweden 
Johanna Eriksson, Ph.D.
Associate MIDD consultant
Pharmetheus
Uppsala, Uppsala Lan, Sweden- JG
Jean-Yves Gillon, Ph.D.
DNDi
Geneve, Geneve, Switzerland - VG
Vishal Goyal, Ph.D.
DNDi
Geneve, Geneve, Switzerland - VS
Vijay Satam, Ph.D.
DNDi
Geneve, Geneve, Switzerland - SR
Stephen Robinson, Ph.D.
DNDi
Geneve, Geneve, Switzerland - HC
Henri Caplain, M.D.
DNDi
Genève, Geneve, Switzerland - IR
Isabela Ribeiro, M.D.
DNDi
Geneve, Geneve, Switzerland - MC
Marylore Chenel, Ph.D.
Pharmetheus AB
Paris, Ile-de-France, France
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: 5-flucytosine (5FC) is used for the treatment of cryptococcal meningoencephalitis (CM). Due to its short plasma half-life and safety profile, it is currently dosed four times a day. This frequent dosing involves high risks of low adherence with potential consequences for both pharmacodynamic effect and toxicity [1,2]. To address this problem and challenges of administration in severely ill non per os patients, a sustained release (SR) pellet formulation is currently under development to decrease frequency and allow naso-gastric tube administration and thus improve the treatment. A model-informed drug development (MIDD) strategy was implemented to inform decisions during the project [3]. In a first step, a PBPK model was developed to support the selection of SR formulations to be studied. From this first work, 3 SR formulation prototypes were selected to be tested for safety and plasma pharmacokinetics (PK) in fasted healthy participants (study 1), along with a commercial immediate-release (IR) tablet. To further support dosing and formulation selection in a subsequent clinical study (study 2: fed study in healthy participants) the legacy PBPK model was updated with the PK data obtained in study 1. The aim of this analysis was to predict the food effect in healthy participants for the SR and IR formulations to suggest a dosing regimen for the study in fed healthy participants.
Methods: PBPK modeling was performed in PK-Sim v.9.1. A legacy PBPK model for 5-FC had previously been developed based on literature data of an IR formulation [4]. The PK data obtained from study 1 was used to further update and refine the legacy model. The final model included a fraction unbound in plasma set to 97% [5] and elimination attributed to glomerular filtration [6]. The intestinal permeability was estimated to a high value to describe the fast Tmax seen for solution [6] and Weibull functions were estimated for the IR and SR formulations to describe the slower absorption for undissolved formulations. In addition, the colonic absorption was decreased to describe the observed PK data. The model was evaluated by visual inspection of the concentration-time profile and comparison of simulated versus observed PK parameters. To predict the food effect on PK for 5-FC, the gastric emptying time (GET) was prolonged in accordance with the available implementation in PK-Sim. The solubility of 5-FC was not considered to be altered by food intake as it is a drug with high water solubility, and thus not expected to be affected by solubilization by bile salts. A therapeutic interval of Cmax not higher than 100 mg/L and Ctrough between 20-70 mg/L was considered in this analysis.
Results: The refined 5-FC PBPK model could well describe the PK data in study 1 (Figure 1), with absolute average fold error (AAFE) values for AUClast and Cmax of 1.13 for both parameters. The SR formulation with the fastest release (formulation D) had the highest bioavailability (62%) of the SR formulations relative to IR tablet in study 1. Prolonged GET enables longer time for absorption and the predicted relative bioavailability for formulation D in fed state increased to 80%, still having the highest bioavailability of the three SR formulations. The estimated dose for treatment D to be within the therapeutic target concentrations with a bi-daily dosing were approximately 5000 mg in fed state and 6000 mg in fasted state (Figure 2). According to simulations, a dose of 6000 mg can be given as a first dose regardless of prandial state (if unknown for unconscious patients when admitted to the hospital) without exceeding the therapeutic Cmax concentration. In addition, the simulations do not support a loading dose to be given together with the SR formulation to achieve comparable PK as the IR formulation, since the simulated difference in time to reach same Cmax as IR was only about 20 min.
Conclusion: The SR formulation D was recommended for development due to the beneficial PK compared to the other two SR formulations. In addition, a dose of 6000 mg was suggested by the simulations to achieve therapeutic target concentrations in fasted state. The need for loading dose for the SR formulation to be comparable to the IR formulation was not supported by modeling. The risk of exceeding the therapeutic interval, as a consequence of uncertainties in prandial state, was predicted to be low even at a higher starting dose (6000 mg). The presented model will be refined based on data from study 2 and applied for the subsequent step in the MIDD strategy, to inform the study design of Phase II.
References: [1] Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. The Journal of antimicrobial chemotherapy 2000; 46(2): 171-9.
[2] Archibald LK, Tuohy MJ, Wilson DA, et al. Antifungal susceptibilities of Cryptococcus neoformans. Emerging infectious diseases 2004; 10(1): 143-5.
[3] EFPIA MID3 Workgroup, Marshall SF, Burghaus R, Cosson V, Cheung SY, Chenel M, DellaPasqua O, Frey N, Hamrén B, Harnisch L, Ivanow F. Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT: pharmacometrics & systems pharmacology. 2016 Mar;5(3):93-122.
[4] Pai MP, Bruce H, Felton LA. Clinical pharmacokinetics of oral controlled-release 5-fluorocytosine. Antimicrobial agents and chemotherapy. 2010 Mar;54(3):1237-41.
Acknowledgements: This project is funded by the European & Developing Countries Clinical Trials Partnership (EDCTP2) programme supported by the European Union, with additional funding from the Swiss Agency for Development and Cooperation (SDC), Médecins Sans Frontières International, and other private foundations and individuals. The findings and conclusions contained herein are those of the authors and do not necessarily reflect positions or policies of the aforementioned funding bodies.
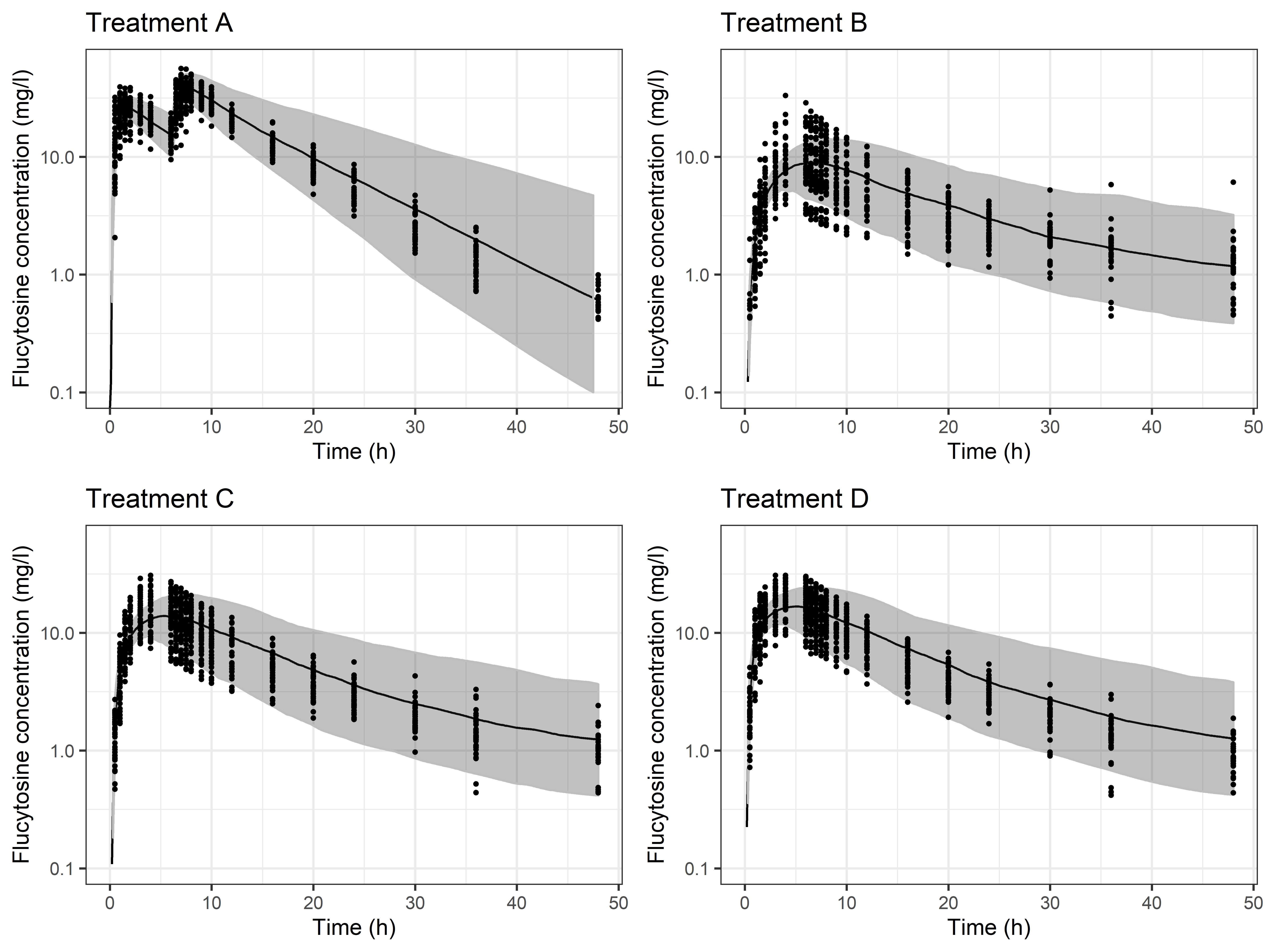 Simulation with final model in fasted state compared to observed data from study 1 on a log-linear scale. Black dots represent the observed data, black line and shaded area represent the median and 5-95% range for the simulation. Treatment A dosing was 1500 mg at t=0 and t=6 and Treatment B-D dosing was 3000 mg at t=0. BLQ values are not displayed.
Simulation with final model in fasted state compared to observed data from study 1 on a log-linear scale. Black dots represent the observed data, black line and shaded area represent the median and 5-95% range for the simulation. Treatment A dosing was 1500 mg at t=0 and t=6 and Treatment B-D dosing was 3000 mg at t=0. BLQ values are not displayed.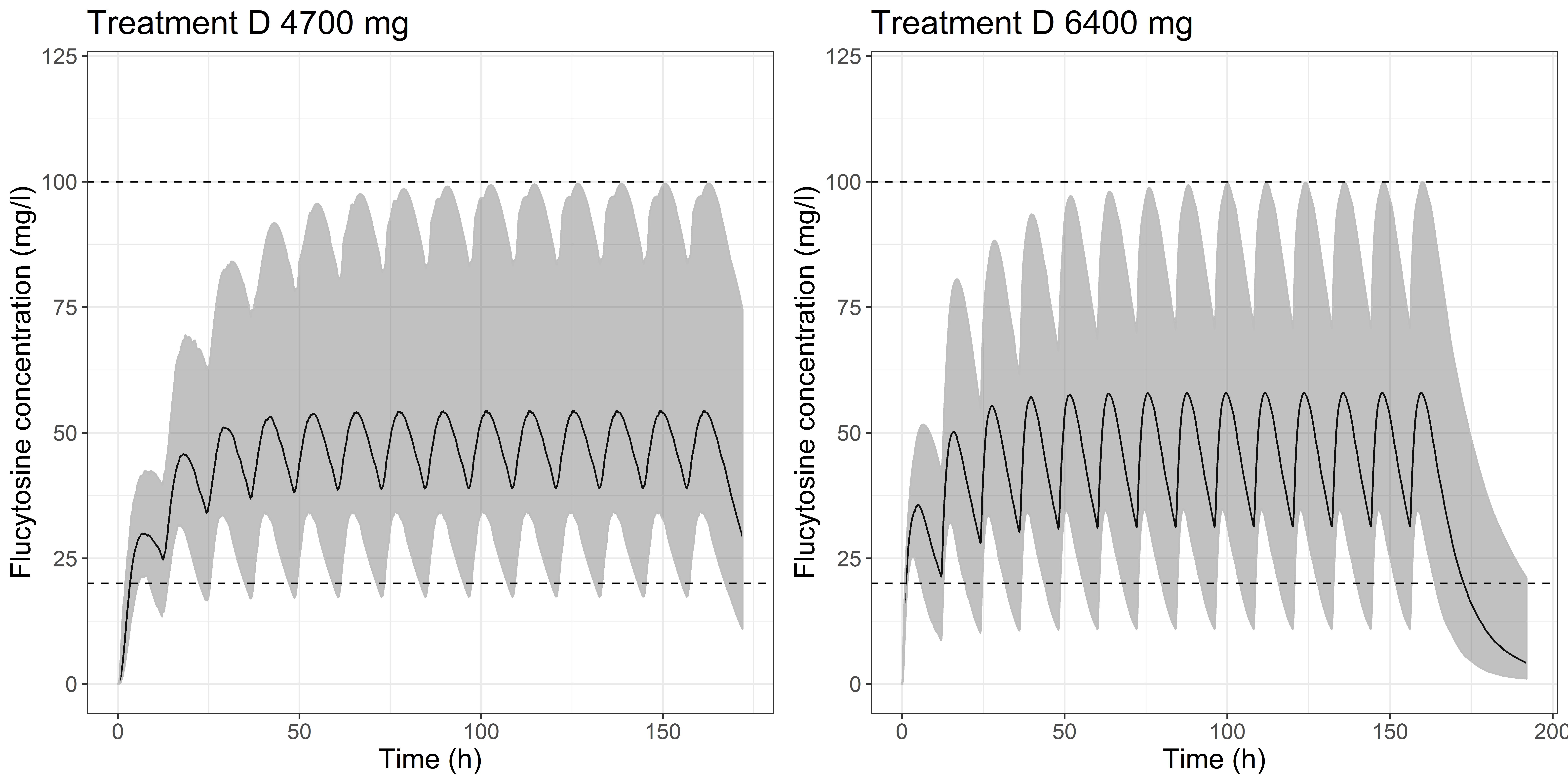 Predictions with base model in fed state and final model in fasted state, where the doses for each prototype were optimized to be within the therapeutic target window 20-100mg/L (dotted lines). Black line and shaded area represent the median and 5-95% range for the prediction.
Predictions with base model in fed state and final model in fasted state, where the doses for each prototype were optimized to be within the therapeutic target window 20-100mg/L (dotted lines). Black line and shaded area represent the median and 5-95% range for the prediction.