Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M1130-08-53) Investigation of Non-specific Adsorption of Lipid Components of the Emulsion-Based Formulations Stored in Glass Vials
Monday, October 23, 2023
11:30 AM - 12:30 PM ET

Erika Patel, MS
Senior Scientist
Merck & Co., Inc.
West Point, Pennsylvania, United States
Erika Patel, MS
Senior Scientist
Merck & Co., Inc.
West Point, Pennsylvania, United States
Tian Lu, Ph.D.
Sr Scientist
Merck & Co., Inc.
Lansdale, Pennsylvania, United States- WS
William J. Smith, Ph.D.
Merck & Co., Inc.
Lansdale, Pennsylvania, United States - PA
Patrick Ahl, Ph.D.
Merck & Co., Inc.
Lansdale, Pennsylvania, United States - LC
Lorenzo Chen, Ph.D.
Merck & Co., Inc.
Lansdale, Pennsylvania, United States - RS
Randy Soukup, Ph.D.
Merck & Co., Inc.
Lansdale, Pennsylvania, United States - PZ
Ping Zhuang, Ph.D.
Merck & Co., Inc.
Lansdale, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Emulsion-based formulations are widely used in the pharmaceutical development because of their long history of use, effectiveness, and established safety. The stability of emulsions is very important during storage. Various analytical approaches are used to evaluate the destabilization of the emulsions due to creaming/sedimentation, coalescence, and Ostwald ripening during the product development. Non-specific adsorption of the formulation components can also affect the stability of the emulsions. Therefore, there is a continuous need to develop the analytical approaches to support the root cause investigation and understand the mechanism of non-specific adsorption. The main purpose of this study is to discuss the mass-balance approach used to investigate the non-specific adsorption of the lipid components of the emulsion formulations stored in glass vials for the stability study. Re-consideration of formulations buffer and excipient to mitigate the non-specific adsorption is also discussed in this study.
Methods: For this study, Oil-in-water emulsion formulation samples stored at elevated temperature (37 °C) and at 4°C in glass vials were used. 4°C samples were used as control samples. Stability of the lipids content were monitored using an ultra high-performance liquid chromatography in combination with a Corona Charged Aerosol Detector (UHPLC-CAD). To quantify the lipid content, lipids were separated using the UHPLC-CAD on a Waters BEH C18 UPLC reversed-phase column. Mobile phases of water and methanol containing acetic acid and triethylamine (pH 5.4) were used for the separation. It is similar to the UHPLC-CAD method conditions described in ref 1. A significant loss (~40%) of lipid components were observed for 1week, 37°C condition samples without any visible signs of degradation (Figure 1 & 2). To investigate the loss observed, sample vials stored at 37 °C and 4 °C for 1 week were emptied completely and rinsed using the assay diluent Ethanol. Rinse solutions were analyzed using UHPLC-CAD to measure the concentrations of the lipid components. Data obtained from the rinse experiments were used to perform the mass balance and recovery calculation of the lipid content. Later, as mitigation approach, several formulation buffers were evaluated and put on stability during formulation development. A formulation buffer with added excipient was used to perform another rinse experiment on 1week, 37°C condition samples of previous stability study. Recovery of lost lipids using new buffer matrix as rinse solution was also calculated via mass balance approach.
Results: Minimum to no adsorption was observed for 1 week 4°C samples used as control samples. For 1 week 37°C sample rinsed using assay diluent (Ethanol), about 43% and 49% of Lipid 1 and Lipid 2 were recovered from the rinse respectively. Total recoveries of 109% and 106% of Lipid 1 and Lipid 2 were calculated with respect to the concentrations observed in T0 sample. This recovery and mass balance data confirmed the loss in the lipid contents were due to the adsorptions of the lipids on the sides of the glass vials used for storage. Based on the results, formulation was further optimized and monitored on stability. 5 weeks accelerated stability study at two different concentrations of lipids showed no significant changes to the lipid concentrations for the new formulation buffer with additional excipients (Figure 3) at 37°C. Upon rinsing the 1week, 37°C stability sample (No excipient in the matrix buffer) using a formulation buffer with added excipient, a recovery of 22% was observed for both lipid components which indicated that addition of excipient into formulation buffer was helpful in mitigation of the nonspecific adsorption.
Conclusion: An efficient and simple approach of mass balance was successfully utilized to investigate the non-specific adsorption of the lipid components in the emulsion formulation. Based on the results, the optimized formulation buffer showed less to almost no loss of lipids than the original formulation buffer. The investigation and mitigation approaches used in this study will be a beneficial contribution to emulsion formulation development applied not only in pharmaceutical industry but also in industries such as food, cosmetics etc.
References: Kinsey C, Lu T, Deiss A, Vuolo K, Klein L, Rustandi RR, Loughney JW. Determination of lipid content and stability in lipid nanoparticles using ultra high-performance liquid chromatography in combination with a Corona Charged Aerosol Detector. Electrophoresis. 2021 Nov 16:10.1002/elps.202100244. doi: 10.1002/elps.202100244. Epub ahead of print. PMID: 34784061; PMCID: PMC8652870.
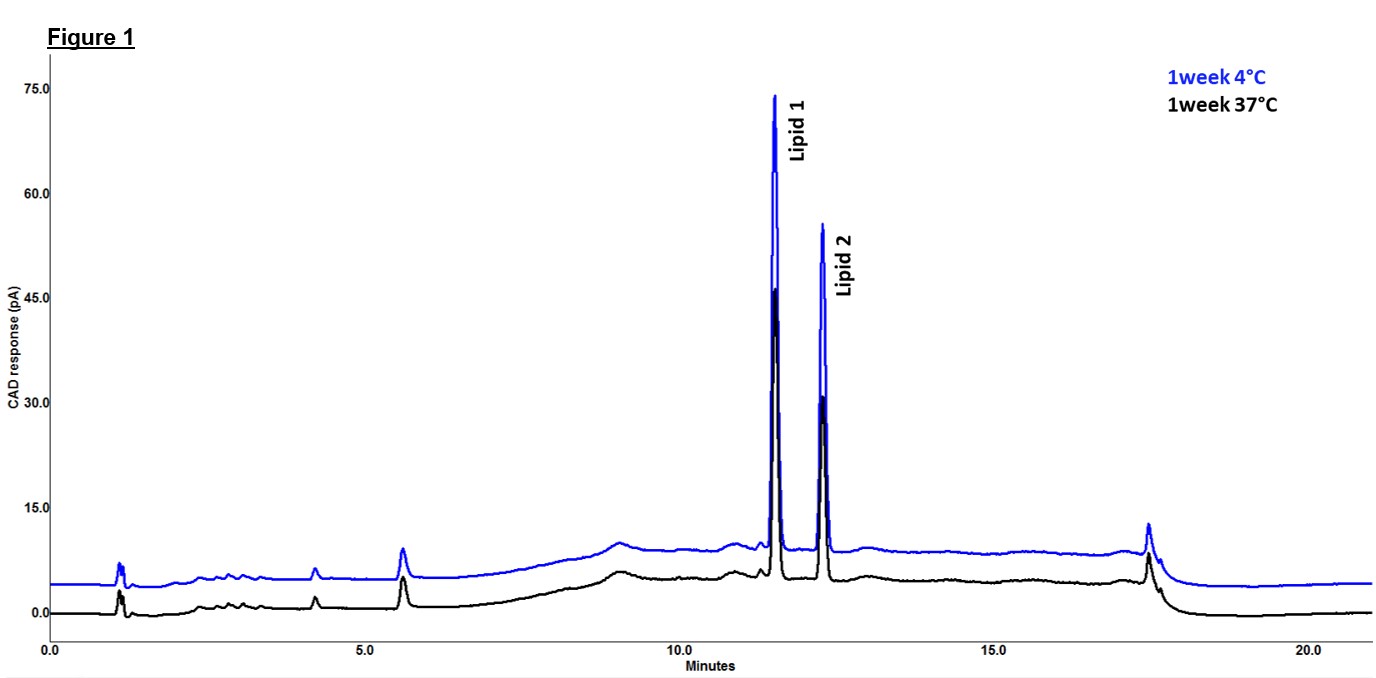 Figure 1: Chromatographic overlay of stability samples stored at 4°C and 37°C for 1 week showing no signs of degradation peaks.
Figure 1: Chromatographic overlay of stability samples stored at 4°C and 37°C for 1 week showing no signs of degradation peaks..jpg) Figure 2: Stability data of the emulsion formulated in excipient-free buffer showing ~40% loss of Lipid 1 and Lipid 2 for the sample stored at 37 °C for 1 week.
Figure 2: Stability data of the emulsion formulated in excipient-free buffer showing ~40% loss of Lipid 1 and Lipid 2 for the sample stored at 37 °C for 1 week.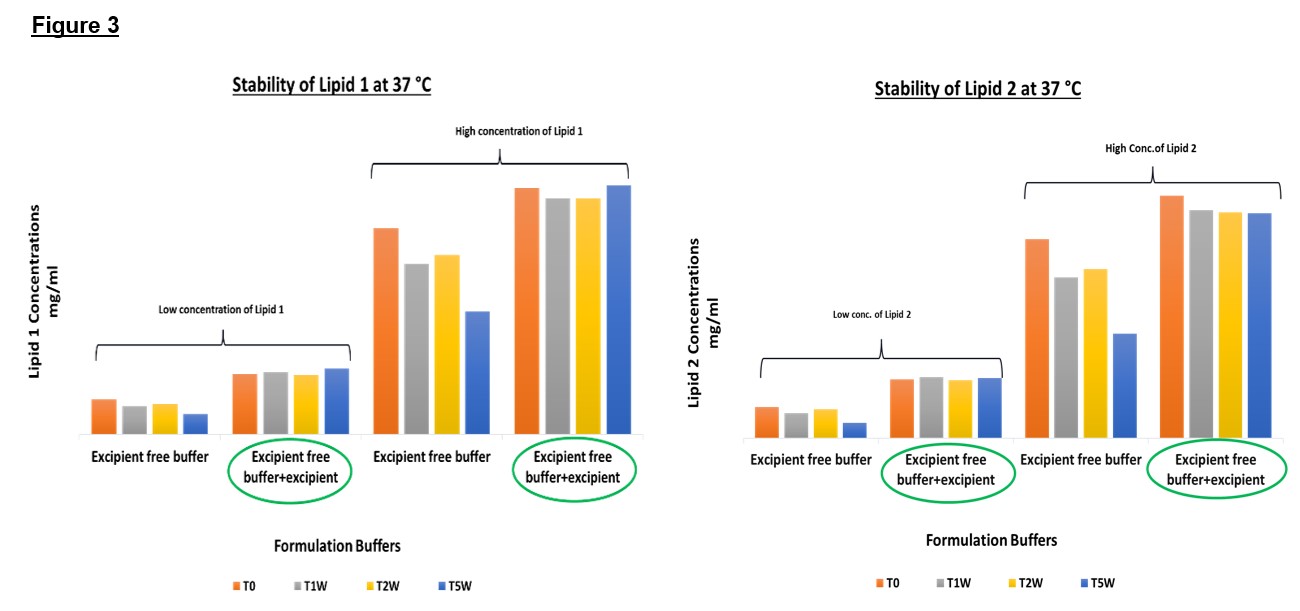 Figure 3: Stability of two different concentrations of Lipid 1 and Lipid 2 formulated in excipient free buffer and in the buffer with added excipient under the storage temperature of 37°C up to 5 weeks.
Figure 3: Stability of two different concentrations of Lipid 1 and Lipid 2 formulated in excipient free buffer and in the buffer with added excipient under the storage temperature of 37°C up to 5 weeks.