Manufacturing and Analytical Characterization - Biomolecular
Category: Poster Abstract
(T1230-08-49) Addressing the Challenges of Industrial Manufacturing of Lipid-Based Complex Injectables: Fine-Tuning of Membrane Fluidity Using Quality-by-Design as a Tool to Enhance Process Effectiveness
Tuesday, October 24, 2023
12:30 PM - 1:30 PM ET

Mariana Biscaia, MS (she/her/hers)
PhD Student
University Coimbra/Bluepharma
Coimbra, Coimbra, Portugal
Mariana Biscaia, MS (she/her/hers)
PhD Student
University Coimbra/Bluepharma
Coimbra, Coimbra, Portugal- AL
Ana Sofia Lourenço, Ph.D. (she/her/hers)
Bluepharma
Coimbra, Coimbra, Portugal - AN
Antonio Nunes, Pharm.D., Ph.D. (he/him/his)
Bluepharma
Coimbra, Coimbra, Portugal - JM
Joao Nuno Moreira, Pharm.D., Ph.D. (he/him/his)
University Coimbra
Coimbra, Coimbra, Portugal - SS
Sérgio Simões, Pharm.D., Ph.D. (he/him/his)
Bluepharma
Coimbra, Coimbra, Portugal
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Over the last three decades, the advantages of lipid-based complex formulations as drug delivery systems have been well-recognized, resulting in at least eight FDA/EMA-approved liposomal products containing remotely loaded small molecular weight drugs. However, from an industrial point-of-view, the manufacturing of these formulations is very time-consuming and energy-demanding due to its intricate multistep nature. A particular challenge at the industrial scale (relative to the laboratory scale) is associated with heating the preparation, often around 60-65ºC, in a timely manner and ensuring thermal homogeneity in the bulk suspension. Hence, using lower temperatures could lead to leaner manufacturing, while reducing the energetic waste. In this respect, it is well established in the literature that remote drug loading should be performed above the liposome’s phase transition temperature. Herein, we challenge this dogma and hypothesize that the fine-tuning of membrane fluidity could be used as a potential tool to enhance and predict drug encapsulation efficiency at much lower temperatures, ultimately leading to a more effective manufacturing process. Accordingly, the current study applies quality-by-design principles to determine the lowest temperature, shortest drug incubation period, and corresponding membrane fluidity, needed to enable efficient remote loading.
Methods: DSPC/CHOL/DSPE-PEG liposomes were prepared by the extrusion method, followed by remote doxorubicin loading using ammonium sulfate as the loading agent. The unencapsulated drug was removed by size exclusion chromatography using sephadex G-50 gel columns equilibrated with HEPES/NaCl buffer, pH 7.2. Total lipid and doxorubicin concentrations were determined through cholesterol quantification using the Liebermann-Burchard reaction and a spectrophotometric method (A492nm), respectively. Loading efficiency was determined using the equation: [drug-to-total lipid final molar ratio/drug-to-total lipid initial molar ratio]x 100. Quality-by-design tools such as Ishikawa diagrams and design of experiments were used to gain a comprehensive understanding of the membrane’s fluidity impact on drug loading efficiency. Membrane fluidity was evaluated by a fluorometric assay using the laurdan probe, an environmentally sensitive solvatochromic probe that, upon incorporation into membranes, exhibits different fluorescence spectra (Ex350nm; Em440 and Em 490) depending on the physical state of the surrounding phospholipids. Membrane fluidity was determined using the equation: 1/[(Em440-Em490)/(Em440+Em490)].
Results: An initial design of experiments was performed in which different incubation temperatures (25, 37, 45 and 65ºC) and periods (15, 30 and 60 min) were tested using high cholesterol (40 mol%) liposomes (with a phase transition temperature of 55ºC). Doxorubicin loading efficiencies above 90% were obtained at 65ºC, regardless of drug incubation time. Particularly remarkable was the efficient drug loading (98%) obtained upon incubation over 60 min at 45ºC, which is 10ºC below the liposome’s phase transition temperature. However, at 37ºC loading efficiency dramatically dropped to values 6.1-fold lower. As these could potentially be explained by small changes in membrane fluidity, an Ishikawa diagram (Figure 1) was used to identify key process and formulation parameters that might impact this attribute. Three critical factors were selected to be studied in a second design of experiments: the surface tension of the isotonic agent used in the extraliposomal environment during drug loading, cholesterol content (mol%) and drug incubation temperature. Results from this design revealed that lowering surface tension and increasing cholesterol content and temperature, increased membrane fluidity (Figure 2), enabling a faster and more efficient drug loading (Figure 3). In fact, for liposomes with high cholesterol content (40 mol%), it was possible to obtain an encapsulation efficiency of 92% following a 30 min incubation at 45ºC in the presence of glycerin, contrasting with the 66% loading efficiency observed in the presence of dextrose (Figure 3). This might be explained by the high membrane fluidity conferred by glycerin (1.1-fold higher than for dextrose), associated with a significantly lower surface tension at 45ºC when compared to dextrose at equal osmotic pressure. Moreover, since only liposomes with a membrane fluidity above 3.8 reached drug loading efficiencies above 90%, data suggested that there is a membrane fluidity threshold that must be reached to enable efficient drug loading. This was confirmed with low cholesterol liposomes (20 mol%), where drug incubation at 45ºC, associated with a membrane fluidity of either 3.5 or 3.7 (for both dextrose and glycerin, respectively) resulted in low loading efficiency (62%). However, at 50ºC, still below the phase transition temperature, membrane fluidity became higher than 3.8 (3.9 for dextrose and 4.5 for glycerin), enabling 95-100% drug loading for all the timepoints tested (15, 30 and 60 min).
Conclusion: Herein, with the support of quality-by-design tools, it was successfully demonstrated that, through fine-tuning of membrane fluidity, it was possible to efficiently encapsulate doxorubicin below the phase transition temperature of the main phospholipid (DSPC) in less than an hour. This could minimize the use of high temperatures during the manufacturing of lipid-based complex injectables which might be advantageous in terms of process efficiency, particularly at large scales.
References: 1. L.D. Mayer, M.J. Hope, P.R. Cullis, «Vesicles of variable sizes produced by a rapid extrusion procedure», Biochim. Biophys. Acta 858 (1986) 161–168.
2. Bolotin, R. Cohen, L.K. Bar, S.N. Emanuel, D.D. Lasic, Y. Barenholz, «Ammonium sulphate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandosomes», J. Liposome Res. 4 (1994) 455–479.
3. Xiong, Q., Wilson, W. K. and Pang, J., «The Liebermann-Burchard reaction: sulfonation, desaturation, and rearrangment of cholesterol in acid». Lipids, 42(1) (2007), 87-96
4. Jay & Hamilton, «Disorder Amidst Membrane Order: Standardizing Laurdan Generalized Polarization and Membrane Fluidity Terms», J Fluoresc 27:1 (2017): 243–49;
5. Söderlund et al., «Comparison of the Effects of Surface Tension and Osmotic Pressure on the Interfacial Hydration of a Fluid Phospholipid Bilayer», Biophysical Journal 85:4 (2003): 2333–41.
Acknowledgements: The authors acknowledge the financial support of the project CInTech-Technological Hub for Innovation, Translation and Industrialization of Complex Injectable Drugs, with the reference n.º C644865576-00000005, co-funded by Component C5 – Capitalisation and Business Innovation under the Portuguese Resilience and Recovery Plan, through the NextGenerationEU Fund.
This work had the financial support of the Portuguese Foundation for Science and Technology (FCT) and Bluepharma – Indústria Farmacêutica S.A, through the Ph.D. research project “Programa de Doutoramento em Empresa FCT: Investigação e Desenvolvimento do Medicamento – Drugs R&D”, in partnership with the Faculty of Pharmacy of University of Coimbra, under the doctoral fellowship grant with the reference PD/BDE/150664/2020.
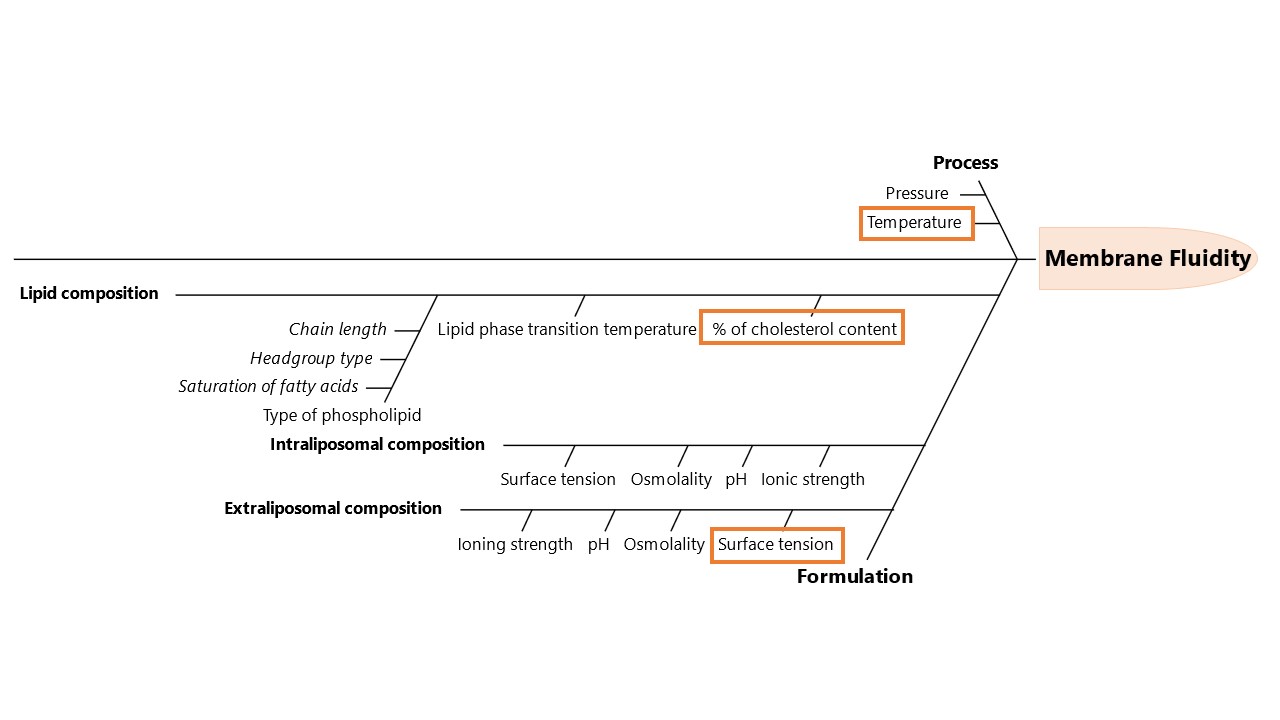 Figure 1: An Ishikawa diagram illustrating process and formulation factors that may have an impact on the liposome’s membrane fluidity.
Figure 1: An Ishikawa diagram illustrating process and formulation factors that may have an impact on the liposome’s membrane fluidity.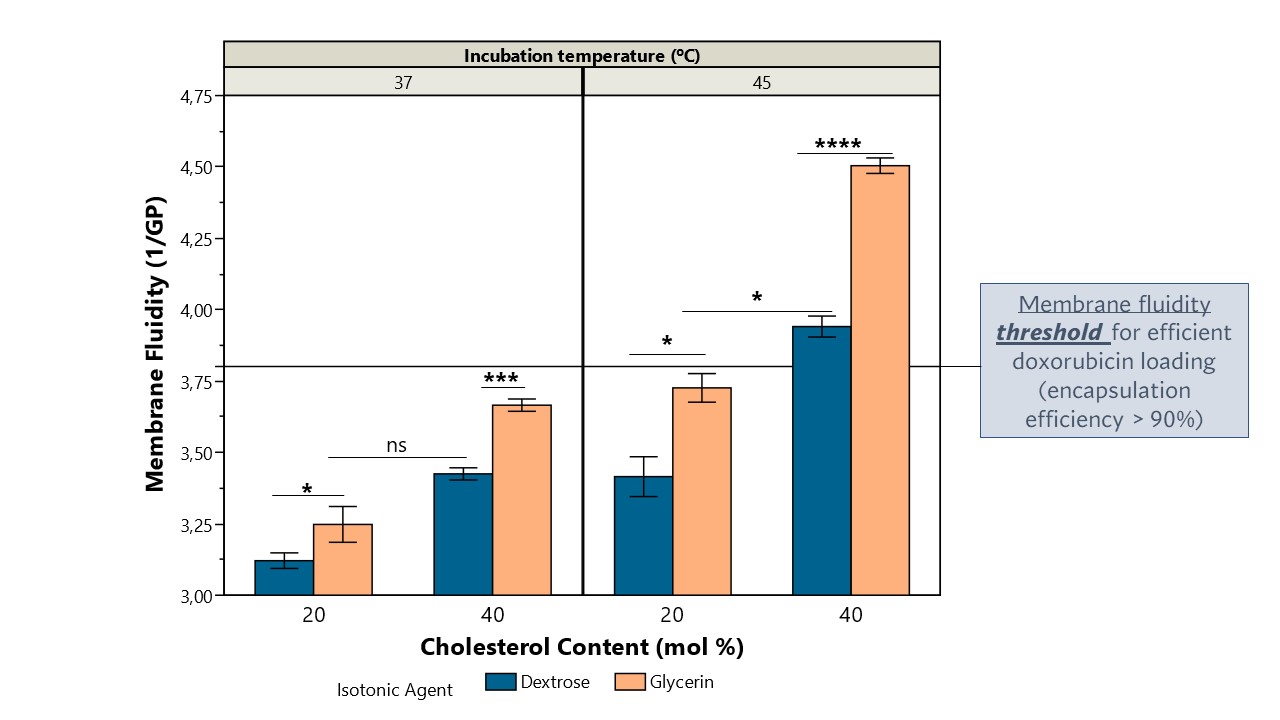 Figure 2: Liposome’s membrane fluidity as a function of cholesterol content (mol%), isotonic agent (dextrose vs glycerin) and incubation temperatures (37 vs 45ºC). Membrane fluidity was determined using the equation: [1/GP], in which GP is the generalized polarization ([(Em440-Em490)/(Em440+Em490)]). Data represent the mean ± SD; the p-value was calculated using Tukey's multiple comparisons test, ****p < 0.001, ***p < 0.001, *p < 0.05, nsp >0.05, n=3.
Figure 2: Liposome’s membrane fluidity as a function of cholesterol content (mol%), isotonic agent (dextrose vs glycerin) and incubation temperatures (37 vs 45ºC). Membrane fluidity was determined using the equation: [1/GP], in which GP is the generalized polarization ([(Em440-Em490)/(Em440+Em490)]). Data represent the mean ± SD; the p-value was calculated using Tukey's multiple comparisons test, ****p < 0.001, ***p < 0.001, *p < 0.05, nsp >0.05, n=3.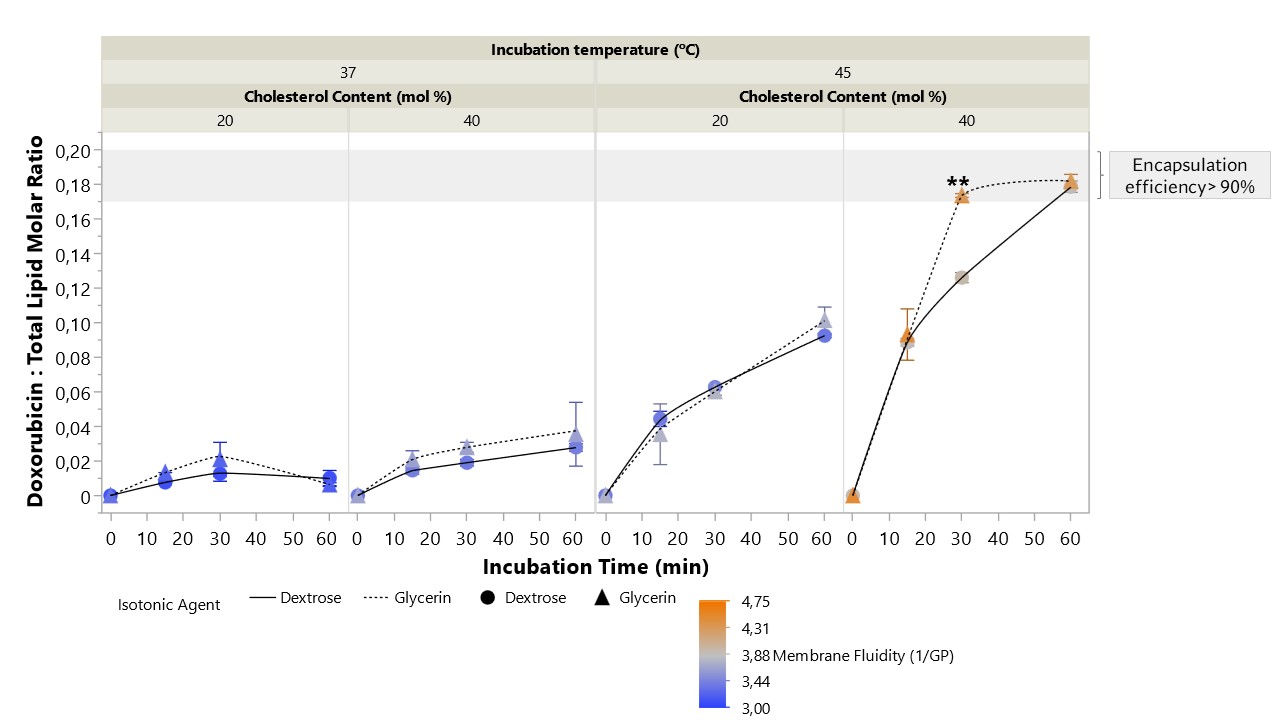 Figure 3: Time course of doxorubicin uptake in DSPC/CHOL/DSPE-PEG liposomes with different % of cholesterol (20 vs 40 mol%) at different temperatures (37 and 45ºC) and using isotonic agents with different surface tension (dextrose – circles and solid line; glycerin- triangles and dashed line). Liposomes were incubated with doxorubicin at a drug:total lipid molar ratio of 0.19:1. The color gradient represents the membrane fluidity of each formulation at the respective incubation temperature. Data represent the mean ± SD; the p-value was calculated using Tukey's multiple comparisons test, **p < 0.01, n=3.
Figure 3: Time course of doxorubicin uptake in DSPC/CHOL/DSPE-PEG liposomes with different % of cholesterol (20 vs 40 mol%) at different temperatures (37 and 45ºC) and using isotonic agents with different surface tension (dextrose – circles and solid line; glycerin- triangles and dashed line). Liposomes were incubated with doxorubicin at a drug:total lipid molar ratio of 0.19:1. The color gradient represents the membrane fluidity of each formulation at the respective incubation temperature. Data represent the mean ± SD; the p-value was calculated using Tukey's multiple comparisons test, **p < 0.01, n=3.