Preclinical and Translational Sciences - Chemical
Category: Poster Abstract
(W1230-10-67) Development of In Vitro In Vivo Correlation for Establishing Clinically Relevant Dissolution Specifications of Lamotrigine ER Tablets
- AS
Abu Bakar Siddique, PhD (he/him/his)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - AS
Abu Bakar Siddique, PhD (he/him/his)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - MS
Maha Shaklah, Pharm.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - OA
Om Anand
US Food and Drug Administration
Silver Spring, Maryland, United States - ML
Min Li, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - FW
Fang Wu, Ph.D. (she/her/hers)
US Food and Drug Administration
Silver Spring, Maryland, United States - KR
Kimberly Raines, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - TO
Thomas O'Connor
US Food and Drug Administration
Silver Spring, Maryland, United States - MA
Muhammad Ashraf, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Ahmed S. Zidan, PhD
Senior Staff Fellow (Pharmacologist)
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Clinically relevant dissolution specifications (CRDS) may be used to understand the impact of formulation and manufacturing process changes on the in vivo performance of drug products. The current study aimed at developing an in vitro in vivo correlation (IVIVC) for establishing a CRDS for lamotrigine matrix based enteric coated ER formulation. Lamotrigine was used as a model Class IIb drug. The pros and cons of using biorelevant media for developing dissolution testing method were evaluated. The validated IVIVC along with in-silico modelling approach of a physiological based biopharmaceutics modelling (PBBM) will be used to identify a safe, clinically relevant dissolution space for the product.
Methods: To develop an IVIVC model, fast, medium, and slow ER lamotrigine 300 mg tablets were manufactured in house. The dissolution of lamotrigine tablets was tested using USP apparatus II & III under various biorelevant and non-biorelevant dissolution conditions. Bio-relevancy of the dissolution methods included the use of simulated gastric, intestinal, and colonic media without enzymes, and mimicking the gastric transit at different pH. In the USP dissolution method III, agitation rate was varied from 5 to 15 rpm to account for differences in GI motility. The plasma concentration time profiles following administration of ER lamotrigine tablets were deconvoluted using multiple deconvolution functions to obtain percent of in vivo drug released. GastroPlus® 9.8.2 simulation platform was used to develop and validate the PBBM and IVIVC models for establishing the CRDS.
Results: Fast, medium, and slow releasing tablets were manufactured. These tablets met all acceptance criteria for dosage uniformity. The results in Figure 1 show the dissolution profiles of these tablets in non-biorelevant and biorelevant dissolution media using USP apparatus II and III. The tablets exhibited relatively lower drug release in USP apparatus III compared to that of USP II. This observation may be attributed to lack of sink conditions in USP apparatus III and supersaturation in view of smaller volume of dissolution medium. The PBBM adequately predicted the Cmax and AUC of the IV and oral IR lamotrigine profiles with a confidence limit more than 95%. Fitting the dissolution data of the ER tablet formulations to the PBBM revealed higher predictability when Loo-Riegelman function was employed compared to numerical or mechanistic deconvolution functions. Having the least prediction error for AUC and Cmax, the best IVIVC model was a 2nd order polynomial function between fraction absorbed and fraction dissolved for all three tablet formulations. The developed IVIVC model met both the internal and external validation criteria for Cmax and AUC per FDA guidance.
Conclusion: This study described a systematic approach to develop an IVIVC using in vitro dissolution data generated under biorelevant and non-biorelevant conditions. This IVIVC may be used to establish CRDS for lamotrigine ER tablets. This CRDS may define a safe dissolution space to support scale-up and post-approval changes.
Acknowledgements: Disclaimer: This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
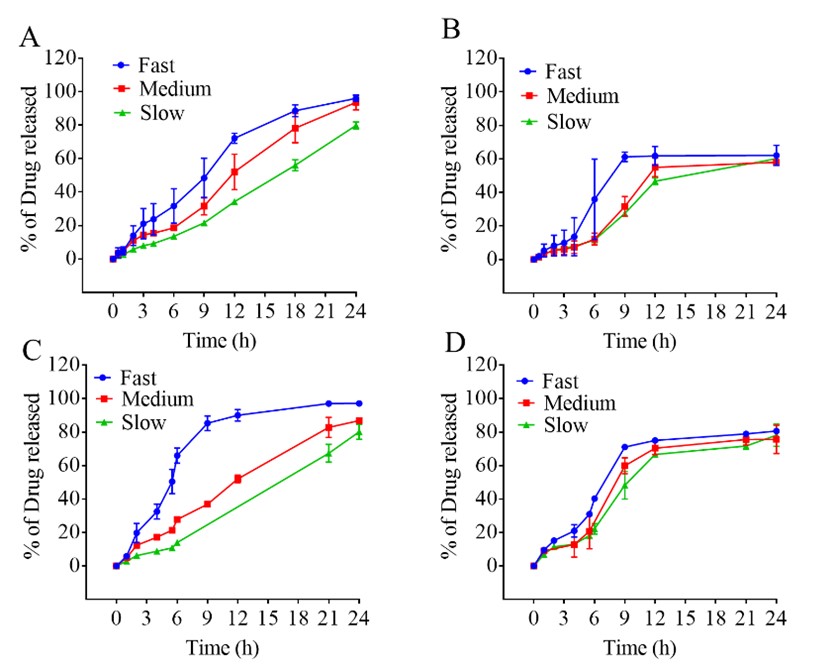 Dissolution profiles of lamotrigine 300 mg fast, medium, and slow-release tablets in non-biorelevant dissolution medium using USP apparatus A) II and B) III. Biorelevant dissolution medium using USP apparatus C) II and D) III.
Dissolution profiles of lamotrigine 300 mg fast, medium, and slow-release tablets in non-biorelevant dissolution medium using USP apparatus A) II and B) III. Biorelevant dissolution medium using USP apparatus C) II and D) III.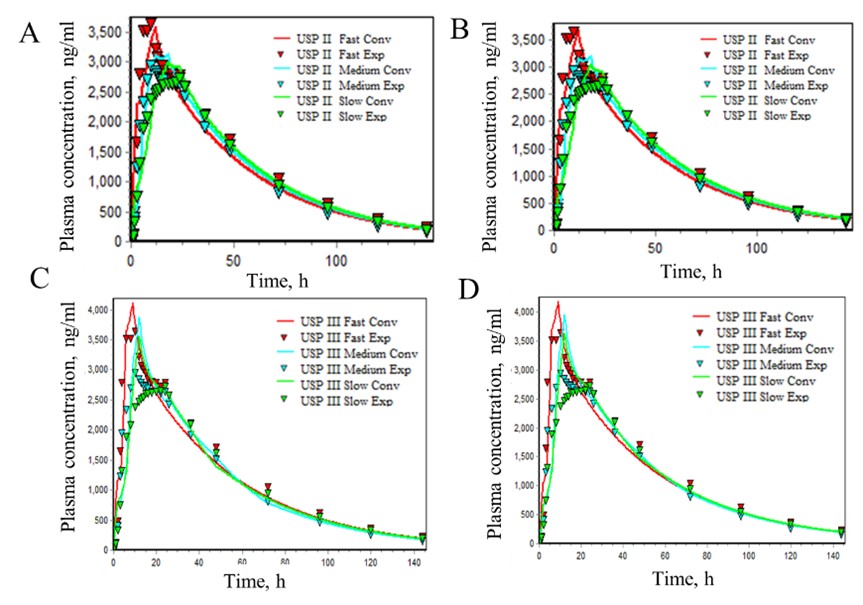 Validation of the IVIVC model using Loo-Reigelman deconvolution method: A and C) Internal validation for dissolution data using USP apparatus II and III in non-biorelevant media respectively. B and D) External validation for dissolution data using USP apparatus II and III in biorelevant media respectively.
Validation of the IVIVC model using Loo-Reigelman deconvolution method: A and C) Internal validation for dissolution data using USP apparatus II and III in non-biorelevant media respectively. B and D) External validation for dissolution data using USP apparatus II and III in biorelevant media respectively.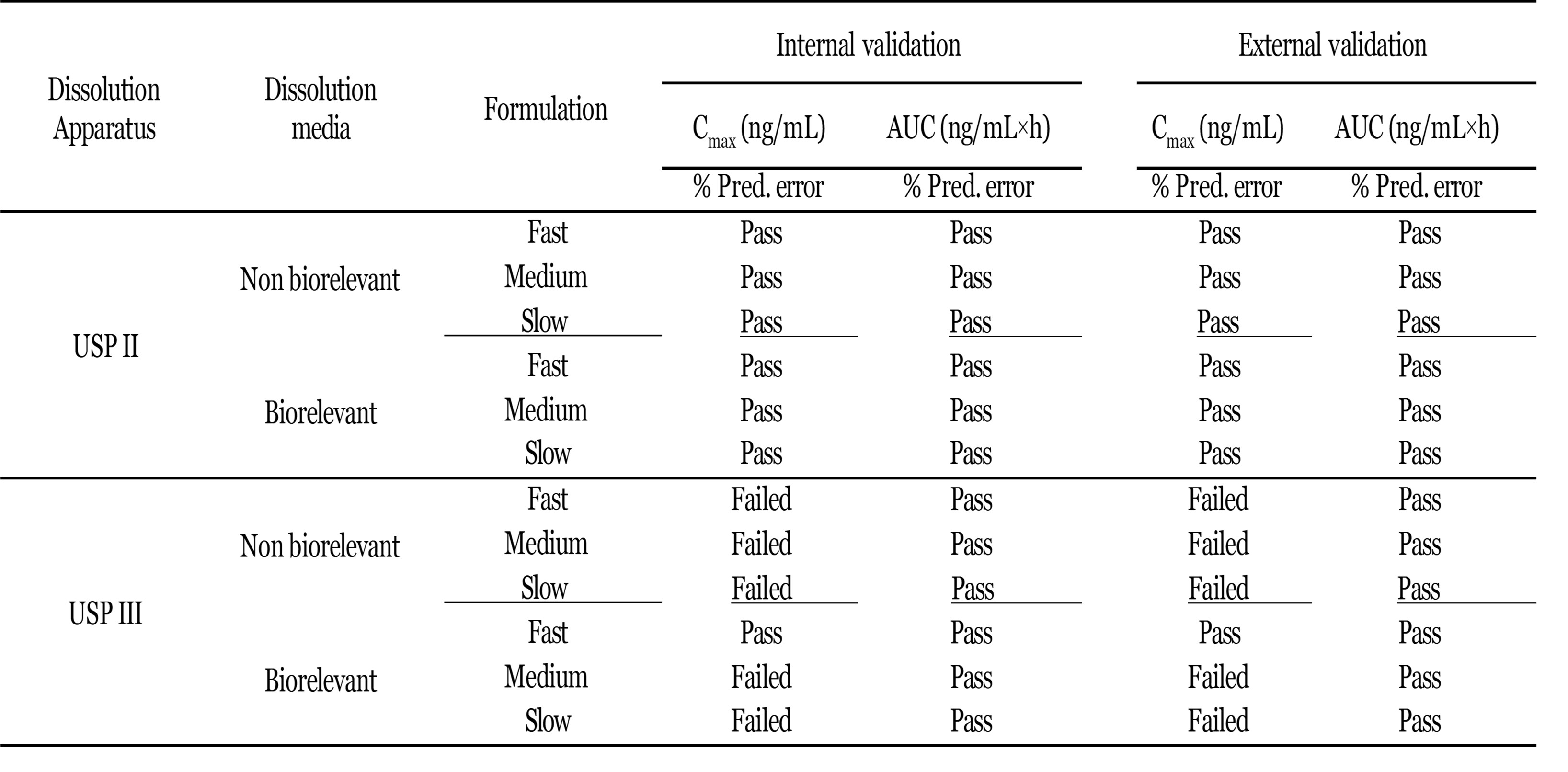 IVIVC fitting summary in USP II and USP III dissolution for lamotrigine ER 300 mg
IVIVC fitting summary in USP II and USP III dissolution for lamotrigine ER 300 mg