Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M1330-08-55) Understanding Release Mechanism and Development of Accelerated Release Tests for Long-Term Intrauterine Systems
- RZ
Rokon U. Zaman, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - RZ
Rokon U. Zaman, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - WS
William Smith, Ph.D.
US Food and Drug Administration
Silver Springs, Maryland, United States - JP
Jin Park, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - JL
Jing Liang, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - XF
Xin Feng, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - FZ
Feng Zhang, Ph.D. (he/him/his)
University of Texas at Austin
Austin, Texas, United States 
Ziyue Zhong, MS (he/him/his)
Research assistant
University of Texas at Austin
Austin, Texas, United States- AC
Andrew G. Clark, Ph.D. (he/him/his)
DigiM Solution LLC
Woburn, Massachusetts, United States - FZ
Fan Zhaobo, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - JZ
Jiwen Zheng, Ph.D.
US Food and Drug Administration
White Oak, Maryland, United States - MA
Muhammad Ashraf, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - XX
Xiaoming Xu, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Several brands of levonorgestrel (LNG) intrauterine systems (IUSs) are commercially available for long-term contraception. The complex product design and its long in vivo application duration present unique challenges for development of generics. To this end, FDA has published a draft product-specific guidance (PSG) on LNG-IUS recommending a combination of in vitro, in vivo/ex vivo studies referencing the brand-name LNG-IUS, Mirena1. The PSG-recommended short-term (1-year) in vivo study which significantly reduced the burden on the clinical evaluation of IUS. In addition, PSG also recommended long term in vitro drug release testing for five years which may be challenging. Therefore, understanding the formulation design and drug release mechanism is critical for developing an accelerated in vitro drug release testing (IVRT) method without changing the drug release mechanism. Accordingly, the short-term goal of this current study is to explore potential accelerated IVRT methods for LNG-IUSs by better understanding the drug release mechanism. The long-term goal is to facilitate development of generic LNG-IUSs by providing more guidance on IVRT for bioequivalence purpose.
Methods: Possible solvents suitable for conducting accelerated drug release studies were selected based on Hanson Solubility Parameter (HSP) analysis. HSP values of the drug and polymer were determined by investigating their solubility in different solvents (Figure 1). Water permeability of the polydimethylsiloxane (PDMS) membrane was investigated utilizing vertical diffusion cells. Disc Shaped Drug Reservoir-Membrane (DR-M) system with a PDMS membrane thickness of 0.3 mm that mimic the drug release mechanism of IUS was fabricated by compression molding (Figure 2 A and B). For DR-M fabrication, a PDMS polymer similar to that of Skyla IUS was selected by using a battery of thermal and mechanical tests. The drug release rates of fabricated DR-M systems in selected solvents were investigated using vertical diffusion cells and fiber optic-based UV-spectroscopy. Partition coefficients of LNG from PDMS in selected solvents were also determined. HSP approach was utilized to explain LNG-IUS accelerated release data from literature2 and inhouse release studies. The impact of drug release on the microstructure of Skyla IUS samples was investigated using artificial intelligence (AI) assisted surface scanning electron microscopy (SEM) and Focused ion beam scanning electron microscopy (FIB-SEM).
Results: The results of solubility and solvent permeation studies revealed that PDMS membrane is impermeable to aqueous solvents, suggesting that drug release from the PDMS membrane of IUS is mediated by partitioning mechanism. The results of drug release studies indicated that DR-M system exhibited decreasing release rate in selected solvents in the following order: 20% tetrahydrofuran (THF) > 20% isopropanol > 20% ethanol > normal saline (Figure 2C). These drug release rates were found to correlate well with the release trend predicted by HSP analysis and the partition coefficients in selected solvents. The faster drug release rate in 20% THF may be attributed to a change in the release mechanism from partitioning to solvent mediated diffusion because PDMS is permeable to THF. The drug release rates were directly correlated with Relative Energy Difference Area (RED-Area) as was the case with previously published release data2. The AI-assisted microstructural analysis of Skyla IUS samples indicated that the volume of inner drug reservoir decreased as drug released from the IUS. This volume reduction in the inner drug reservoir was about 40% when 25% drug was released. The decrease in the volume of inner drug reservoir may be possibly explained by the pressure exerted on the inner drug reservoir by the outer elastic membrane. The particle size distribution of drug in pre-release and partial release IUS samples was found to be similar (Figure 3). The similarity in drug particle size distribution in pre- and partial release IUS samples suggested that the release medium did not come in contact with drug in the inner reservoir due to the impermeable PDMS membrane.
Conclusion: Membrane-mediated drug partitioning appears to be the main mechanism of drug release from the IUS due to the impermeability of PDMS membrane to water. The experimentally determined accelerated drug release rates and partition coefficients in selected solvents followed the same order as predicted by HSP analysis. HSP values of drug, polymer, and the solvent along with partition coefficients may be used to predict the release behavior of drug from the polymer and may help in developing accelerated IVRT. Work is in progress to understand the implications of drug release on microstructure changes in the LNG-IUS to elucidate drug release mechanism.
References: 1. U.S. Food and Drug Administration. Draft product-specific Guidance on Levonorgestrel intrauterine device, 52 mg. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021225.pdf.
2. Bao Q, Zou Y, Wang Y, Kozak D, Choi S, Burgess DJ. Drug release testing of long-acting intrauterine systems. Journal of Controlled Release 2019; 316:349-58.
Acknowledgements: This work was supported by funding from the FDA Office of Women’s Health. Drs. Zaman, Smith, and Park were supported in part by the Oak Ridge Association of Universities (ORAU) and the Oak Ridge Institute of Science and Education (ORISE), through an agreement between the U.S. Department of Energy and the U.S. FDA. Disclaimer: This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
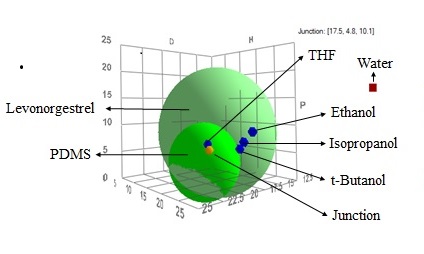 Figure 1. Overlay of HSP solubility spheres of levonorgestrel and PDMS
Figure 1. Overlay of HSP solubility spheres of levonorgestrel and PDMS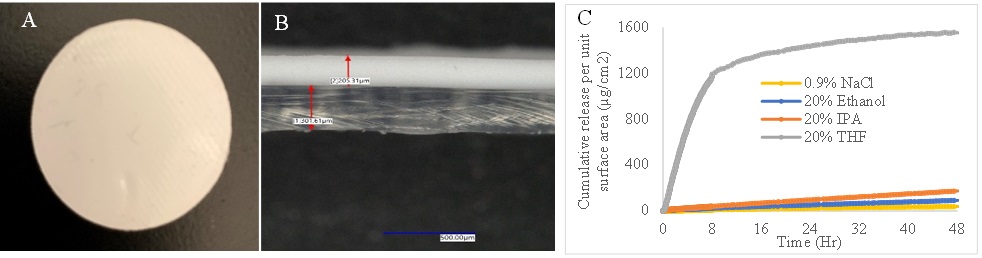 Figure 2. A. Drug Reservoir-Membrane (DR-M) system. B. Cross section of DR-M system.
Figure 2. A. Drug Reservoir-Membrane (DR-M) system. B. Cross section of DR-M system. C. Drug release in different solvents form DR-M system.
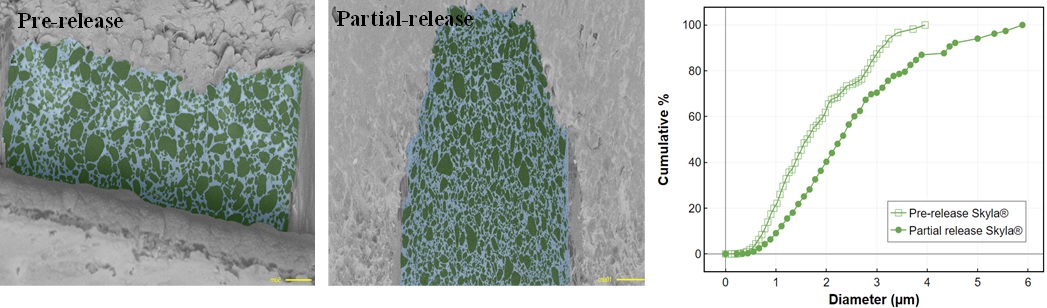 Figure 3. Similar drug concentrations in pre-release (A) and partial release (B) IUS samples as revealed by AI segmentation. C. Comparable LNG size distributions between pre-release and partial release samples.
Figure 3. Similar drug concentrations in pre-release (A) and partial release (B) IUS samples as revealed by AI segmentation. C. Comparable LNG size distributions between pre-release and partial release samples.