Formulation and Delivery - Biomolecular
Category: Poster Abstract
(T1430-01-01) Osimertinib Immunoliposomes as Inhaled Therapy for Non-small Cell Lung Cancer Treatment
Tuesday, October 24, 2023
2:30 PM - 3:30 PM ET
- AD
Apoorva Daram (she/her/hers)
St. John's University
Fresh Meadows, New York, United States - AD
Apoorva Daram (she/her/hers)
St. John's University
Fresh Meadows, New York, United States - SS
Shruti Sawant (she/her/hers)
Purdue University
Queens, New York, United States - VP
Vasudha Prithipaul, B.S. (she/her/hers)
St. John's University
Queens, New York, United States - NK
Nitesh Kunda, Ph.D.
St. John's University
New York, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Lung cancer is one of the most prevalent causes of cancer mortalities globally, of which non-small cell lung cancer (NSCLC) accounts for the majority ( >85%) of those cases. Tyrosine Kinase Inhibitors (TKI) have been extensively used to treat NSCLC with epidermal growth factor receptor (EGFR) overexpression. Osimertinib (OB), a third-generation irreversible TKI, is an approved therapy for NSCLC patients bearing the EGFR T790M mutation. Eventually, resistance to EGFR-TKI therapy due to triple mutations (sensitizing mutations, T790M and C797S) will become a significant challenge. Therefore, there is a need to overcome the resistance, which can be effectively achieved by targeting the EGFR with anti-EGFR monoclonal antibodies. In this study, osimertinib was encapsulated in liposomes, and the surface of the liposomes was modified for conjugation with an anti-EGFR monoclonal antibody, cetuximab (CTX), to enhance drug delivery and anti-tumor efficacy. Further, pulmonary administration of liposomes was utilized for safe and efficacious delivery of the drug to the target site, limiting systemic toxicity.
Methods: In the present study, osimertinib liposomes were prepared using the thin film hydration method followed by a conjugation reaction between the N-terminal of the antibody and the carboxylic group present on the liposomal surface to obtain immunoliposomes (conjugated OB liposomes) for pulmonary administration. The prepared immunoliposomes were characterized for particle size, polydispersity, zeta potential, and antibody conjugation efficiency. To further characterize the solid state of the entrapped drug, powder X-ray diffraction (PXRD) studies were performed. The stability of the antibody on the liposomal surface was assessed using polyacrylamide gel electrophoresis (SDS-PAGE) and surface plasma resonance (SPR). The in-vitro drug release profile for the immunoliposomes was studied in phosphate-buffered saline at pH 7.4. The aerosolization performance of the formulation was evaluated using a Pari LC nebulizer device on a Next Generation Impactor (NGITM). The in-vitro cytotoxicity was evaluated using MTT assay on the NSCLC cell line, H1975. Additionally, clonogenic and wound healing assays were performed to further assess the effectiveness of the immunoliposomes. The stability studies for the immunoliposomes were performed for 4 weeks upon storage at refrigerated conditions (4˚C).
Results: These immunoliposomes exhibited a particle size of around 150 nm with a zeta potential of -2.69±0.98mV and an antibody conjugation efficiency of 87.53±2.84% (Fig. 1A). The disappearance of intense peaks in the XRD diffractogram for the unconjugated and conjugated OB liposomes when compared to the free drug indicated the successful encapsulation of the drug into the liposomes (Fig. 1B). The gel electrophoresis for the antibody and conjugated liposomes resulted in good separation of the antibody, and the bands were detected at 100 kDa which corresponds to the molecular weight of cetuximab, indicating that the protein was stable after the preparation (Fig. 2A). The data from surface plasma resonance demonstrated similar binding kinetics for the immunoliposomes and free antibody. The conjugated liposomes demonstrated an initial burst release of 53.42% in 10h followed by complete release within 48h (Fig. 2B). Additionally, in-vitro aerosolization studies performed using NGI demonstrated a mass median aerodynamic diameter of 3.22±0.12µm and a high fine particle fraction of 88.43±0.38%, implying the deposition of the formulation in the lung respiratory airways (Fig. 2C). The in-vitro studies on H1975 cells displayed higher cytotoxicity for immunoliposomes with ~1.7 and ~1.2-fold reduction in IC50 values compared to free drug and unconjugated osimertinib liposomes, respectively (Fig. 3A). Furthermore, the immunoliposomes displayed significant suppression in tumor cell migration over 48h compared to free drug and unconjugated osimertinib liposomes (Fig. 3B). The clonogenic assay for the immunoliposomes revealed a trend in decrease when compared to the free drug and unconjugated liposomes (Fig. 3C). Further stability studies on the immunoliposomes proved their stability when stored at 4˚C for 4 weeks.
Conclusion: The EGFR-targeting inhalable immunoliposomes were successfully formulated, and the in-vitro cell culture studies showed that they can potentially contribute to greater anti-tumor efficacy for non-small cell lung cancer treatment.
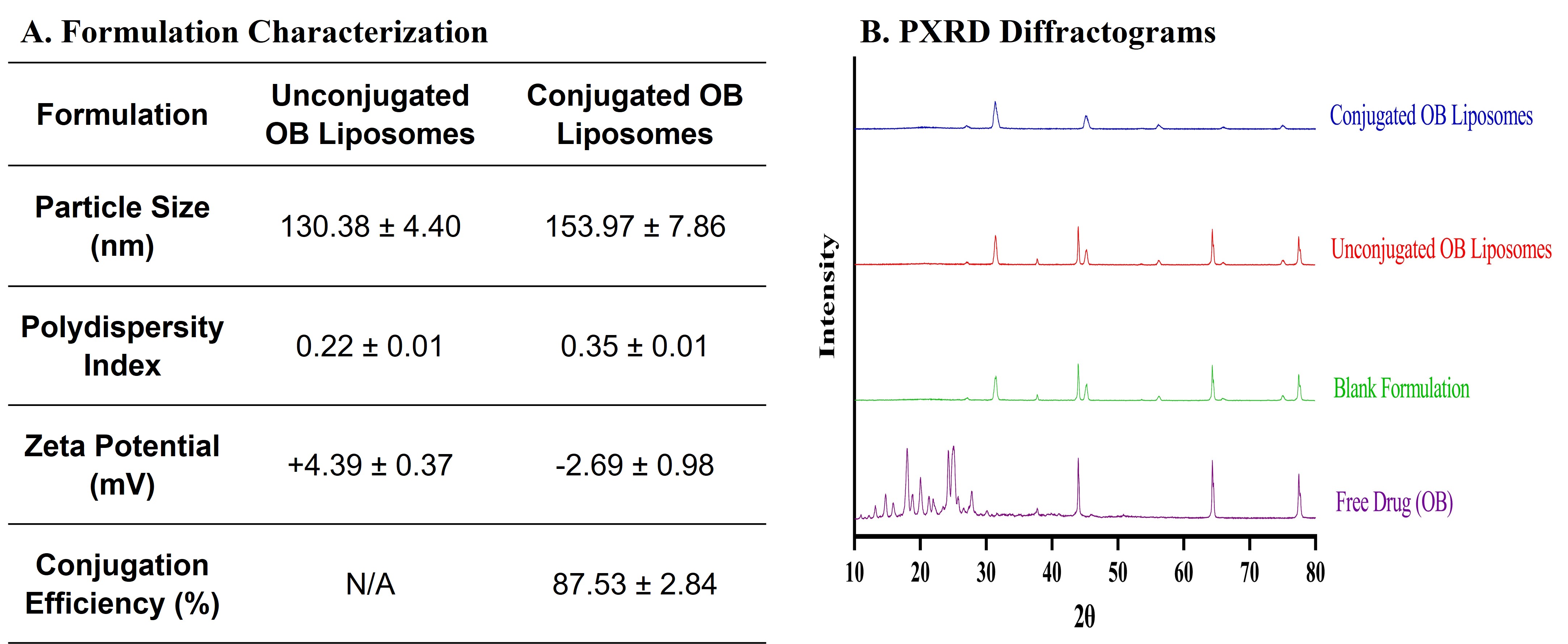 Figure 1. Immunoliposome formulation development. A. Physiochemical characterization of Osimertinib Immunoliposomes (Conjugated OB Liposomes). Data represents mean ± SD (n = 5). B. The XRD diffractogram of OB, blank formulation, unconjugated OB liposomes, and conjugated OB liposomes.
Figure 1. Immunoliposome formulation development. A. Physiochemical characterization of Osimertinib Immunoliposomes (Conjugated OB Liposomes). Data represents mean ± SD (n = 5). B. The XRD diffractogram of OB, blank formulation, unconjugated OB liposomes, and conjugated OB liposomes.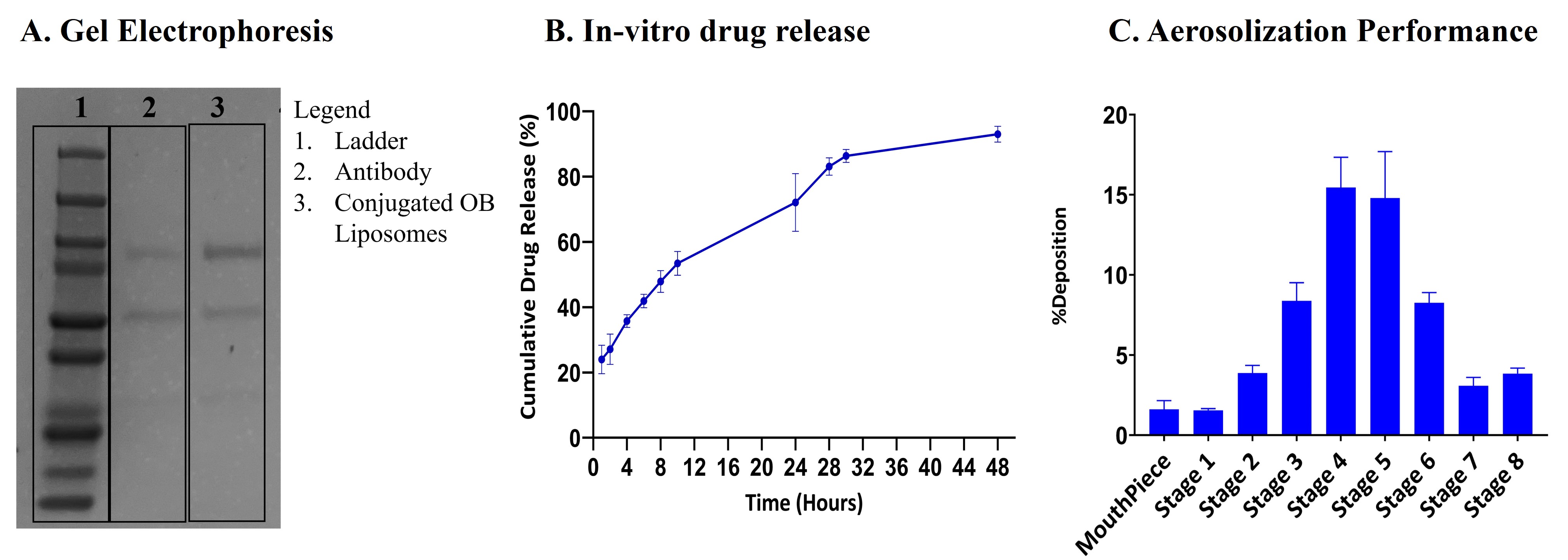 Figure 2. Immunoliposome characterization. A. Gel electrophoresis/Coomassie blue staining of free antibody and conjugated OB liposome samples. B. Cumulative release profile for OB from immunoliposomes in phosphate buffer saline (PBS), pH 7.4. Data represents mean ± SD (n = 4) C. In-vitro aerosol deposition profile represented as percent of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3)
Figure 2. Immunoliposome characterization. A. Gel electrophoresis/Coomassie blue staining of free antibody and conjugated OB liposome samples. B. Cumulative release profile for OB from immunoliposomes in phosphate buffer saline (PBS), pH 7.4. Data represents mean ± SD (n = 4) C. In-vitro aerosol deposition profile represented as percent of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3)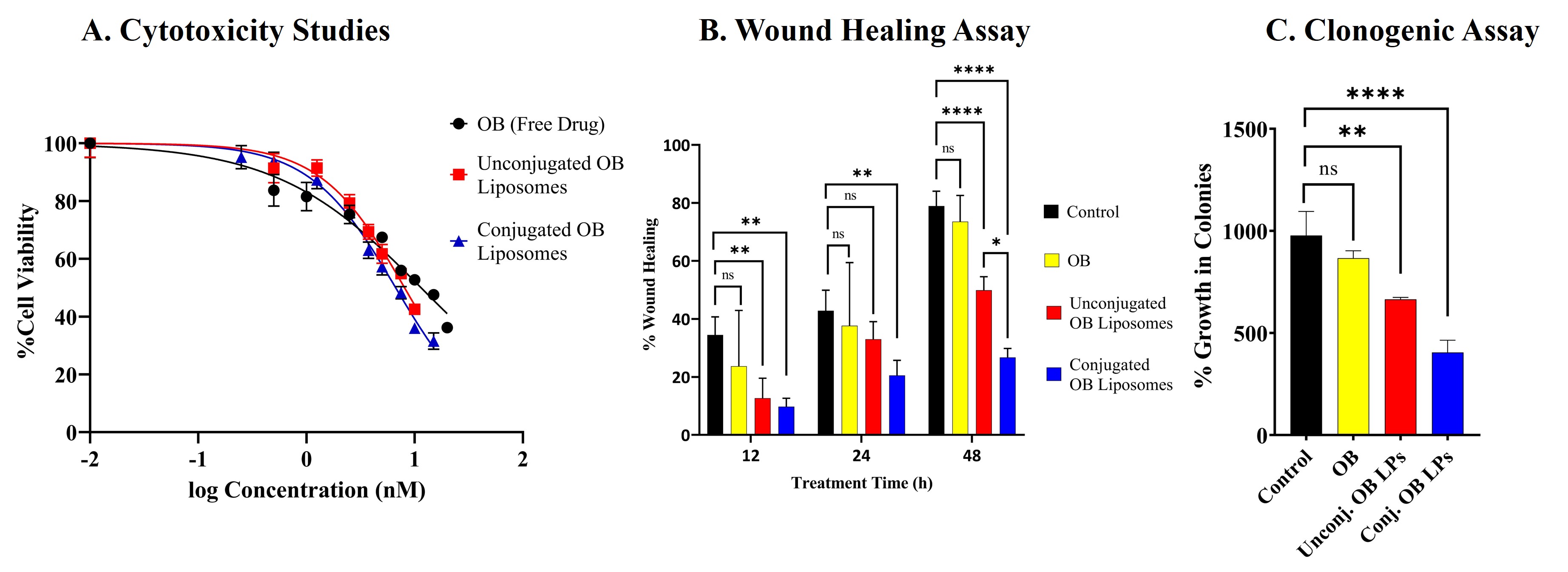 Figure 3. Cytotoxicity and Migration Assay. A. Cytotoxicity studies after 72h treatment with OB, unconjugated and conjugated OB liposomes determined using MTT assay in H1975 cell line. B. Scratch assay analysis of H1975 cell line, shown as % wound healing over time, after treatment with OB, unconjugated and conjugated OB Liposomes. C. Clonogenic assay analysis after 72h of OB, unconjugated and conjugated OB liposomes. Data represents mean ± SD (n = 3)
Figure 3. Cytotoxicity and Migration Assay. A. Cytotoxicity studies after 72h treatment with OB, unconjugated and conjugated OB liposomes determined using MTT assay in H1975 cell line. B. Scratch assay analysis of H1975 cell line, shown as % wound healing over time, after treatment with OB, unconjugated and conjugated OB Liposomes. C. Clonogenic assay analysis after 72h of OB, unconjugated and conjugated OB liposomes. Data represents mean ± SD (n = 3)