Formulation and Delivery - Chemical
Category: Poster Abstract
(M1030-11-71) Dual Functionalized Polymeric Nanoparticles Encapsulating Emtricitabine and Bictegravir for Delivery Across the Blood Brain Barrier for the Management of HIV Associated Neurocognitive Disorders (HAND)
Monday, October 23, 2023
10:30 AM - 11:30 AM ET
- RT
Riddhi Trivedi, BS (she/her/hers)
Graduate student
North Dakota State University
Fargo, North Dakota, United States - RT
Riddhi Trivedi, BS (she/her/hers)
Graduate student
North Dakota State University
Fargo, North Dakota, United States - JS
Jagdish Singh, Ph.D. (he/him/his)
North Dakota State University
Fargo, North Dakota, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Human immunodeficiency virus (HIV) being a neurotropic virus can easily cross the blood-brain barrier, in turn leading to a myriad of neurocognitive dysfunctions which are termed as HIV-associated neurocognitive disorder (HAND). These dysfunctions vary in severity ranging from mild to severe impairment of cognitive abilities. The prevalence of these neurocognitive impairments is seen in 39-70% of HIV-positive patients. The blood-brain barrier prevents the anti-retroviral drugs to reach the brain and maintain therapeutically relevant concentrations for a prolonged period. The brain cells are recognized as the primary reservoir for this virus, thus making the central nervous system (CNS) a vital source of relapse and re-infection. This is believed to be the root cause of HIV-associated neurocognitive impairment. To address this problem, a brain-targeted controlled-release delivery system is needed which can effectively and efficiently deliver anti-retroviral drugs to the brain parenchyma. Hence, in this study, the anti-retroviral drugs Emtricitabine and Bictegravir, loaded functionalized poly (lactic-co-glycolic acid) nanoparticles have been fabricated that can provide controlled drug release in the brain and potentially eradicate the viral depot. These nanoparticles were surface modified with mannose which can target GLUT-1 transporters present on the blood-brain barrier. In addition to this, cell-penetrating peptides, Penetratin or Rabies virus glycoprotein-9R (RVG-9R), were also attached to the surface to enhance cell penetration. These dual-functionalized nanoparticles were tested in-vitro for their cytocompatibility, cellular uptake, and transport across the BBB. Furthermore, the efficacy of this therapy was tested against Eco HIV-infected microglia cells.
Methods: To incorporate high pay loads of both the hydrophilic and hydrophobic drugs, double emulsion technique was optimized to prepare these nanoparticles. The resulting formulation was characterized for its size, zeta potential, and polydispersity index (PDI) using dynamic light scattering (DLS). The nanoparticles were functionalized with cell-penetrating peptide (PLGA-PEG-penetratin/ PLGA-PEG-RVG-9R) and ligand (PLGA-PEG-mannose). The in-vitro release and entrapment efficiency of the nanoparticles were determined through RP/HPLC-UV. The functionalized nanoparticles were also assessed for their cytocompatibility and cellular uptake in bEnd.3 and microglia cells. The hemolytic activity of the functionalized and plain nanoparticles was investigated against rat RBCs. The transporting ability of the formulation was assessed through in-vitro blood-brain barrier model. The ability of this formulation to reduce the viral depot in-vitro, was determined using Eco-HIV infected microglia cells. The viral load was quantified using a p24 ELISA kit.
Results: The optimized dual functionalized dual drug loaded nanoparticles had uniform size distribution and spherical morphology, shown in the TEM images. The nanoparticles had particle size and zeta potential of 235.14± 14.6 nm and -11.29± 1.84mV, respectively with a PDI of 0.086 ± 0.019. The entrapment and loading of Bictegravir were found to be 86.3 ± 9.7% and 8.7 ± 0.98% respectively, while Emtricitabine had the entrapment and loading of 34.3 ± 4.2 % and 14.4 ± 2.3%, respectively. The nanoparticles displayed controlled release of the two drugs, with 90.3 ± 10.7 % Emtricitabine and 85 ± 4.7 % Bictegravir released in 10 days. The cell viability studies confirmed that the prepared formulations were non-toxic to bEnd.3 and glial cells. The maximum cellular uptake was seen after 6 hours of incubation with treatment. The percent hemolysis seen with these nanoparticles was less than 10 % indicating that the formulations are safe for intravenous administration. The modified nanoparticles showed significantly higher cellular uptake and transport across in vitro blood-brain barrier (BBB) model as compared to plain nanoparticles. The in-vitro efficacy study showed that the dual functionalized (PenMan) treated group had significantly lower p24 level as compared to the free drugs treated group.
Conclusion: The double emulsion PLGA nanoparticles had uniform size distribution. The modification of the nanoparticles with mannose and Penetratin (PenManNP) displayed superior cellular uptake, transport across in-vitro BBB barrier model, and efficacy against Eco-HIV infected cells while having good biocompatibility with RBCs, bEND.3 cells and primary glial cells. These results indicate that the dual functionalized polymeric nanoparticles can effectively deliver both drugs across the blood-brain barrier to the brain in a controlled release fashion to thwart the HIV reservoir and manage HIV-associated neurocognitive disorder (HAND).
Acknowledgements: This research was supported by the National Institutes of Health (NIH) Grants R01 AG051574, RF1 AG068034 and 3RF1AG068034-01A1S1.
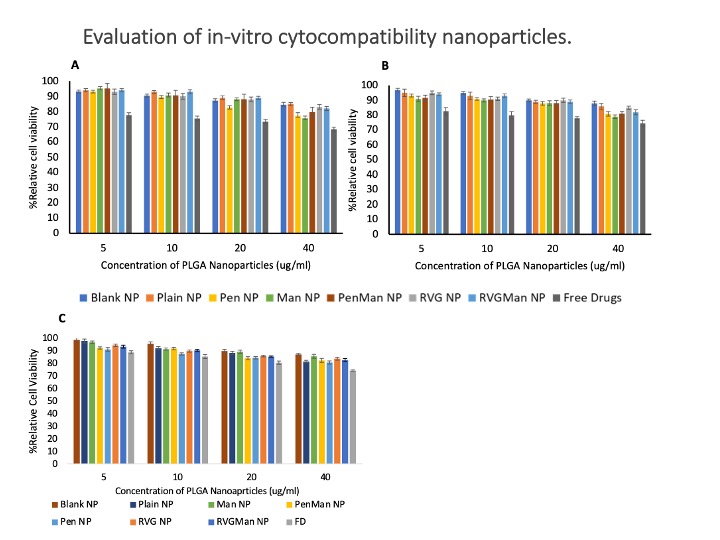 : In-vitro cytocompatibility of different PLGA nanoparticle formulations and free drugs in (A) brain endothelial cells, (B) primary astrocytes and (C) microglia cells. The cellular viability of free drug and PLGA at different concentrations was evaluated upon exposure for 24 h Data represented as mean ± S.D. (n = 6).
: In-vitro cytocompatibility of different PLGA nanoparticle formulations and free drugs in (A) brain endothelial cells, (B) primary astrocytes and (C) microglia cells. The cellular viability of free drug and PLGA at different concentrations was evaluated upon exposure for 24 h Data represented as mean ± S.D. (n = 6). 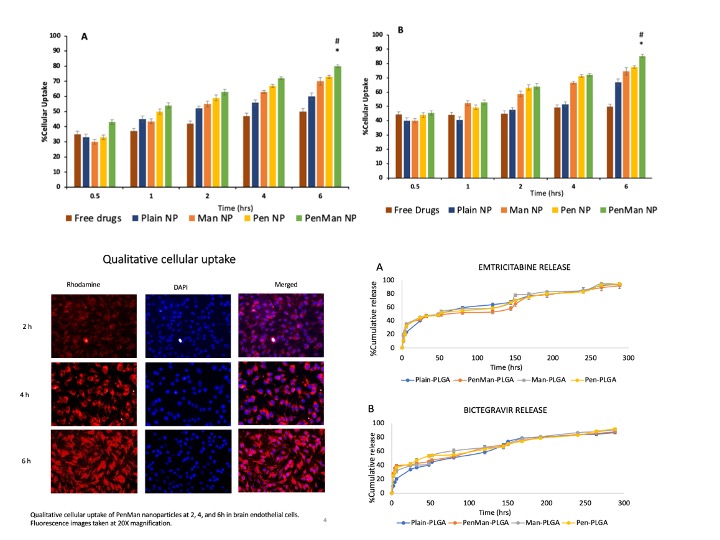 Quantitative cellular uptake of different PLGA nanoparticles and free drugs in bEnd.3 cells (A) and primary astrocytes (B) at 0.5, 1, 2, 4, and 6h. Data represented as mean ± S.D. (n = 6). At 6 h, drug concentration in the cells was significantly higher than all the earlier time points when treated with PenMan NP *(p < 0.05), # PenMan NP was significantly higher compared to other formulations at 6 h. In-vitro release of Emtricitabine (A) and Bictegravir (B) from PLGA Nanoparticles. Formulations were prepared using 40% w/w and 10% w/w drug/polymer ratio, respectively. Data represented as mean ± S.D. (n = 6). No significant difference in drug release was noted between the formulations at all time points.
Quantitative cellular uptake of different PLGA nanoparticles and free drugs in bEnd.3 cells (A) and primary astrocytes (B) at 0.5, 1, 2, 4, and 6h. Data represented as mean ± S.D. (n = 6). At 6 h, drug concentration in the cells was significantly higher than all the earlier time points when treated with PenMan NP *(p < 0.05), # PenMan NP was significantly higher compared to other formulations at 6 h. In-vitro release of Emtricitabine (A) and Bictegravir (B) from PLGA Nanoparticles. Formulations were prepared using 40% w/w and 10% w/w drug/polymer ratio, respectively. Data represented as mean ± S.D. (n = 6). No significant difference in drug release was noted between the formulations at all time points.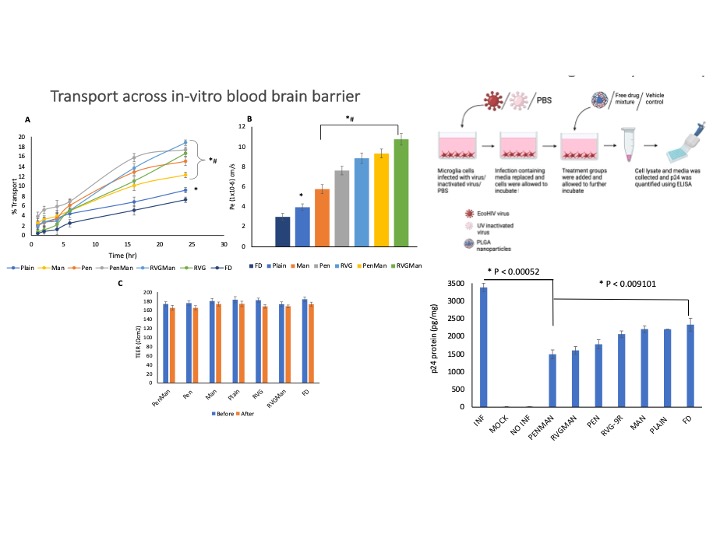 Transport through BBB model over a period of 24 h. (B) Endothelial cell permeability coefficient (Pe, expressed in 1x10-6 cm/s). (C) Measurement of TEER of BBB model before and after 24 h of transport study upon incubation with different PLGA nanoparticles formulations and free drug. Data represented as mean ± S.D. (n = 4). Statistically significant differences (p < 0.05) is shown as (*) with Free drug and (#) Plain nanoparticles. In-vitro viral suppression after treatment with different PLGA nanoparticles and free drug. Data represented as mean ± S.D. (n = 4). Statistically significant differences shown in graph.
Transport through BBB model over a period of 24 h. (B) Endothelial cell permeability coefficient (Pe, expressed in 1x10-6 cm/s). (C) Measurement of TEER of BBB model before and after 24 h of transport study upon incubation with different PLGA nanoparticles formulations and free drug. Data represented as mean ± S.D. (n = 4). Statistically significant differences (p < 0.05) is shown as (*) with Free drug and (#) Plain nanoparticles. In-vitro viral suppression after treatment with different PLGA nanoparticles and free drug. Data represented as mean ± S.D. (n = 4). Statistically significant differences shown in graph.