Formulation and Delivery - Chemical
Category: Poster Abstract
(W1230-01-05) Brain Targeted Delivery of Cannabidiol and Brain Derived Neurotrophic Factor (BDNF) for Attenuation of Inflammation and Promotion of Neurogenesis for the Management of Alzheimer’s disease
Wednesday, October 25, 2023
12:30 PM - 1:30 PM ET
- BC
Bivek Chaulagain, MS
North Dakota State University
Fargo, North Dakota, United States - BC
Bivek Chaulagain, MS
North Dakota State University
Fargo, North Dakota, United States - JS
Jagdish Singh, Ph.D. (he/him/his)
North Dakota State University
Fargo, North Dakota, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The purpose of this study is to deliver the anti-inflammatory drug cannabidiol in combination with BDNF to the brain for the effective management of AD. For the dual delivery of cannabidiol and BDNF gene, liposomes will be employed. The liposomes will be surface functionalized with mannose, a GLUT-1 receptor ligand widely expressed in endothelial cells of BBB, and penetratin to circumvent the BBB.
Methods: Liposomes were prepared by lipid film hydration method using DOPE: DOPTAP: DSPE PEG2000k: Cholesterol at a molar ratio of 45:45:8:2. DSPE PEG Man or DSPE PEG Penetratin were used at 4% molar ratio each for functionalization. Cannabidiol was loaded at 10% w/w of total lipid weight and the drug was estimated using HPLC. Further, BDNF was complexed with chitosan (N: P ratio 5) and added to liposomes during hydration. Zetasizer was used for the physical characterization of liposomes. Release studies were performed using a 12-14 kD dialysis membrane with 0.5 % Tween-80 (PBS pH 7.4) as a release medium. The cellular uptake was estimated after 4 hours using HPLC. DNAse protection assay was performed through an agarose gel electrophoresis. Finally, in vitro, transfection was performed in the IMG cell line using GFP as reporter plasmid. Anti-inflammatory study of the formulation was performed in an IMG cell line stimulated by 10 ng/ml LPS for 24 hours. TNF α and IL-1β protein expression was estimated using ELISA while their relative gene expression was estimated using qPCR. In vitro co-culture BBB model was developed in 24 well-culture inserts using b.END and primary astrocytes and the amount of cannabidiol transported across the barrier were estimated using HPLC.
Results: Plain and functionalized cannabidiol liposomes prepared using the lipid film hydration method resulted in a size of less than 170 nm with homogenous distribution (PDI < 0.2). More than 80% of cannabidiol was encapsulated in each formulation (Figure 1, I). The qualitative and quantitative uptake revealed maximum uptake in 4 hours whereas Pen Man modified liposomes showed significantly higher uptake in IMG cell, b.END cell and primary astrocytes (p < 0.05) compared to plain liposomes (Figure 1, II). The formulation released 81.76 ± 6.16 % of cannabidiol over 7 days (Figure I, III). More than 80% of BDNF plasmid was complexed with the gene. The encapsulated gene was protected against the enzymatic degradation of DNase (Figure I, V). The formulation successfully expressed the pGFP in IMG cells after 48 hours of transfection (Figure 1, VI). All liposomal formulations were able to reduce the protein and gene expression of TNFα and IL-1β compared to free drug and dual-modified liposomes significantly reduced both TNFα and IL-1β protein and gene expression (p < 0.05) (Figure 2). In vitro co-culture model of BBB was developed and its integrity was assessed using TEER measurement and NaF permeation (Figure 3). The transport of cannabidiol liposomes across the BBB in 24 hours showed Pen Man liposomes have significantly higher transport across the barrier compared to plain liposomes (p < 0.05).
Conclusion: Brain-targeted liposomes were successfully formulated encapsulating cannabidiol that released the drug for a week. The formulation also encapsulated the BDNF gene, protected the gene against exonucleases, and expressed the reporter protein in the IMG cells. It also reduced the protein and gene expression of pro-inflammatory cytokine TNFα and IL-1β in LPS inflamed IMG cells. Dual-modified liposomes showed significantly higher permeation across the in vitro BB co-culture models exhibiting the efficient brain-targeting potential of the formulation. To demonstrate its neurogenesis activity and cognitive enhancement role, further studies will be performed in relevant AD mouse models.
Acknowledgements: This research was supported by the National Institute of Health (NIH) grant R01 AG051574, RF1 AG068034, and 3RF1AG068034-01A1S1.
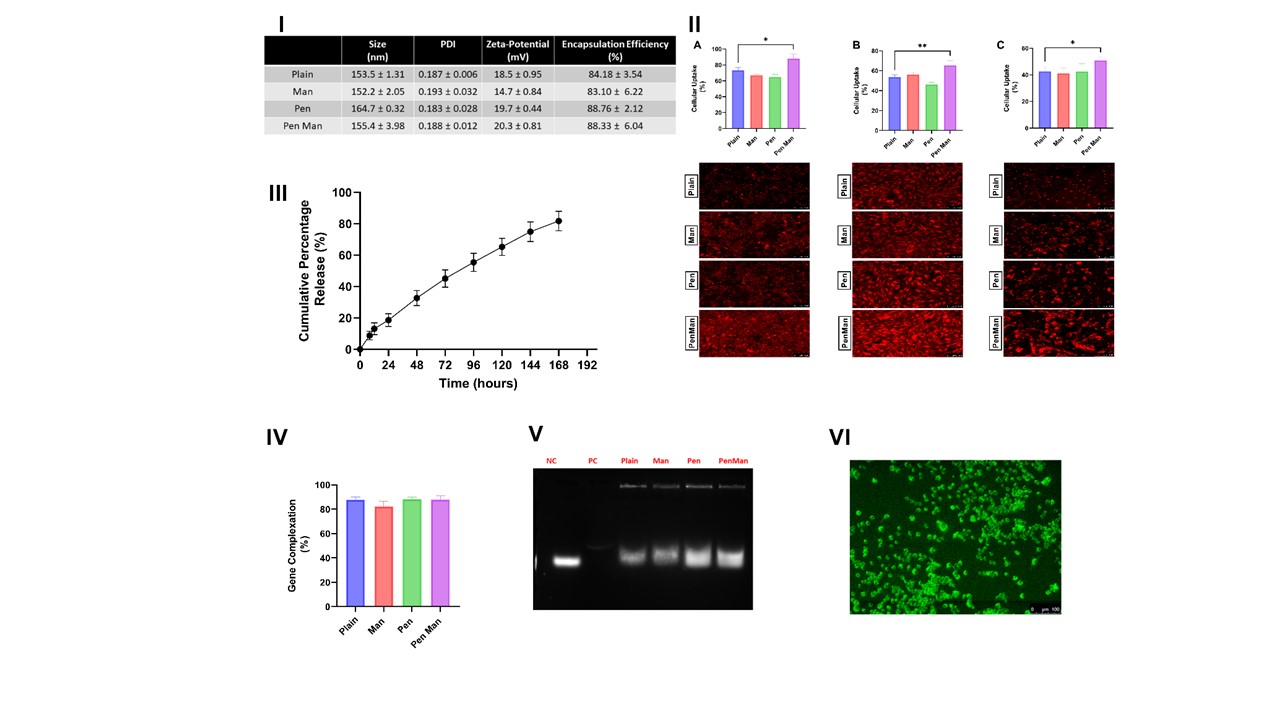 Figure 1: Physiochemical characterization of cannabidiol liposomes for their ability to load cannabidiol and plasmid DNA I) Size, PDI, zeta-potential and encapsulation efficiency of plain and functionalized cannabidiol liposomes II) Quantitative and qualitative cellular uptake of cannabidiol liposomes in A) IMG cell B) b.END cell C) Primary astrocytes III) Cumulative percentage drug release of cannabidiol liposomes for one week IV) Gene complexation of plain and functionalized cannabidiol liposomes estimated using Hoechst 33342 dye V) Agarose gel electrophoresis demonstrating DNase protection assay of plain and functionalized cannabidiol liposomes VI) GFP expression in IMG cell 48 hours after transfection with cannabidiol liposomes. The data represents mean ± SD (n=4) (* p < 0.05)
Figure 1: Physiochemical characterization of cannabidiol liposomes for their ability to load cannabidiol and plasmid DNA I) Size, PDI, zeta-potential and encapsulation efficiency of plain and functionalized cannabidiol liposomes II) Quantitative and qualitative cellular uptake of cannabidiol liposomes in A) IMG cell B) b.END cell C) Primary astrocytes III) Cumulative percentage drug release of cannabidiol liposomes for one week IV) Gene complexation of plain and functionalized cannabidiol liposomes estimated using Hoechst 33342 dye V) Agarose gel electrophoresis demonstrating DNase protection assay of plain and functionalized cannabidiol liposomes VI) GFP expression in IMG cell 48 hours after transfection with cannabidiol liposomes. The data represents mean ± SD (n=4) (* p < 0.05)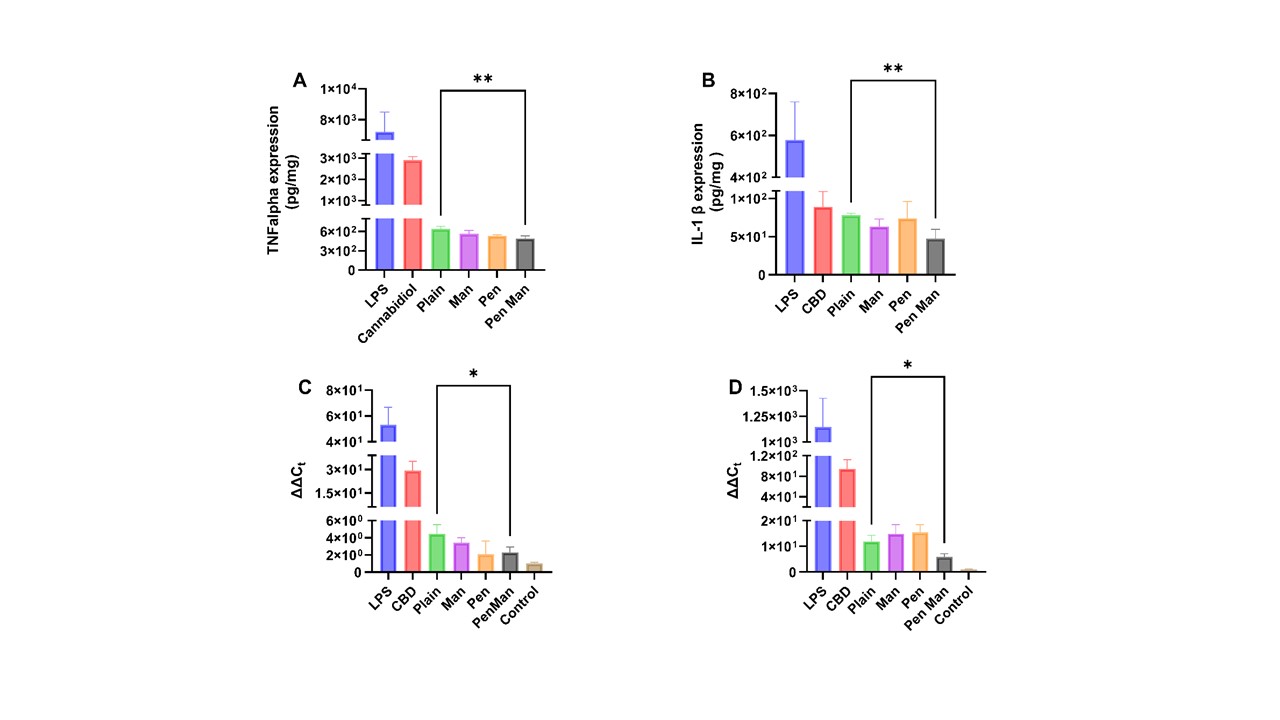 Figure 2: Anti-inflammatory property of cannabidiol liposomes in Immortalized Microglia (IMG) cell line stimulated by 10 ng/ml LPS for 24 hours. The treatment consists of free cannabidiol or cannabidiol liposomes equivalent to 10μM of cannabidiol A) Protein expression of TNF-α estimated using ELISA kits B) Protein expression of IL-1 β estimated using ELISA Kits C) Relative gene expression of TNF-α estimated using qPCR. D) Relative gene expression of IL-1β estimated using qPCR. The data represents mean ± SD (n=4) (* p < 0.05)
Figure 2: Anti-inflammatory property of cannabidiol liposomes in Immortalized Microglia (IMG) cell line stimulated by 10 ng/ml LPS for 24 hours. The treatment consists of free cannabidiol or cannabidiol liposomes equivalent to 10μM of cannabidiol A) Protein expression of TNF-α estimated using ELISA kits B) Protein expression of IL-1 β estimated using ELISA Kits C) Relative gene expression of TNF-α estimated using qPCR. D) Relative gene expression of IL-1β estimated using qPCR. The data represents mean ± SD (n=4) (* p < 0.05)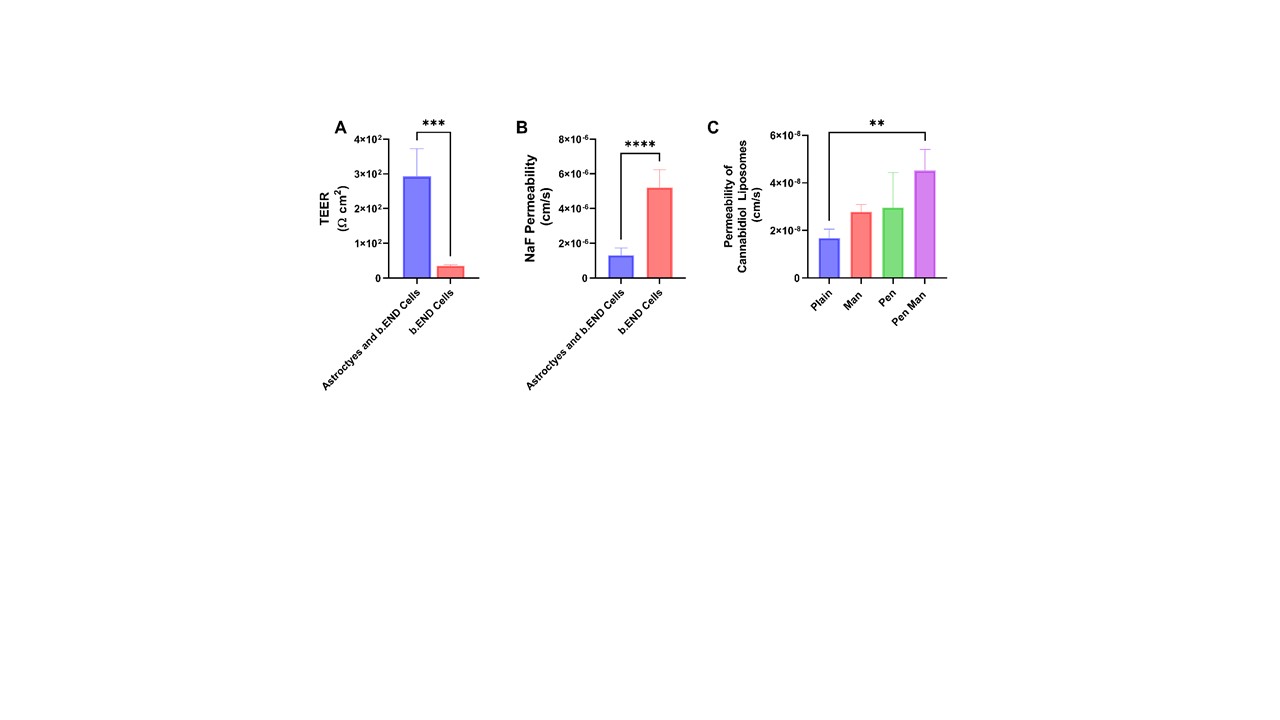 Figure 3: Integrity of in vitro BBB co-culture model and transport of cannabidiol liposomes across the barrier a) TEER of BBB as measured by Epithelial Voltohmeter (EVOM) b) Integrity of BBB as measured by Sodium Fluorescein permeability across the co-culture barrier C) Transport of cannabidiol liposomes across the barrier. The data represents mean ± SD (n=4) (* p < 0.05)
Figure 3: Integrity of in vitro BBB co-culture model and transport of cannabidiol liposomes across the barrier a) TEER of BBB as measured by Epithelial Voltohmeter (EVOM) b) Integrity of BBB as measured by Sodium Fluorescein permeability across the co-culture barrier C) Transport of cannabidiol liposomes across the barrier. The data represents mean ± SD (n=4) (* p < 0.05)