Formulation and Delivery - Chemical
Category: Poster Abstract
(M1430-02-14) Transdermal Delivery of Cannabidiol from Marketed Cannabidiol Patches
- NM
Nisarg Modi, PhD (he/him/his)
Director R&D
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - MJ
Mohammad Shajid Ashraf Junaid, Ph.D.
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - TL
Tamanna Lather, Ph.D. (she/her/hers)
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - YL
Yuliya Levintova, Ph.D. (she/her/hers)
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - MB
Marina Borovinskaya, Ph.D. (she/her/hers)
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - RP
Roda Plakogiannis, Pharm.D. (she/her/hers)
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States - FP
Fotios Plakogiannis, Ph.D. (he/him/his)
Transdermal Research Pharm Laboratories LLC
Long Island City, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The upward trend in the legalization of cannabinoids has garnered much scientific interest in cannabinoid research. Cannabidiol (CBD) is a non-psychoactive cannabinoid. This compound has been reported to be helpful in treating various diseases, such as glaucoma, seizures, and various dermatological conditions. Due to the easy availability of CBD, many non-pharmaceutical companies have brought CBD patches to market. The FDA has circulated warning letters to some of the manufacturing companies for not containing relevant amounts of CBD, as claimed. This research aims to evaluate transdermal delivery from several marketed CBD patches and compare permeation profiles with a CBD transdermal patch formulation developed in-house.
Methods: In this study, a 5% w/w CBD transdermal matrix patch was developed in-house and used as a control. Three marketed CBD patches (Commercial patches 1, 2, and 3) were evaluated. The patches were cut into 7cm2 pieces for the experiments. The drug from these patches was extracted using a 1:1 mixture of Isopropyl alcohol and ethanol. The extraction samples were then analyzed using a validated HPLC-UV method to evaluate the drug content in patches. Vertical Franz diffusion cells were used for in-vitro drug permeation studies. Dermatomed human cadaver skin was used for assessing transdermal delivery of CBD from the marketed patches. The receptor media was 0.5% w/w Brij™ O20 in 10 mM phosphate buffered saline. The skin temperature was maintained at 32o C. The patches were applied on the skin which was clamped between the donor and the receptor chambers of the Franz diffusion cells. The effective permeation area on the skin was 1.76 cm2. Receptor samples were collected at 0, 24, 48, 72, 96, 120, 144, and 168 hr. The collected receptor samples were analyzed using a validated reversed-phase HPLC-UV method.
Results: The amount of CBD that was present in control, commercial patch 1, commercial patch 2, and commercial patch 3 amounted to 652.67 ± 31.63 µg/cm2, 750.41 ± 61.06 µg/cm2, 747.37 ± 41.41 µg/cm2, 769.84 ± 36.73 µg/cm2 respectively. The marketed patches claim 20mg naturally extracted CBD per patch. In our assay we found the amount of CBD present to be slightly below the claimed 20 mg. The results show each patch contained 18.76 ± 1.53. mg, 18.684 ± 1.04 mg, and 19.246 ± 0.93 mg CBD for commercial patches 1, 2, and 3 respectively. The three marketed and the control patches were tested for CBD transdermal delivery on a 7-day in-vitro permeation study. At the end of the 7 days, the average cumulative amount of CBD delivered through dermatomed human cadaver skin from commercial patches 1, 2, and 3 were 1.65 ± 2.86 µg/cm2, 2.98 ± 5.16 µg/cm2, 2.46 ± 4.27 µg/cm2 respectively. The control delivered a significantly higher amount of CBD (55.75 ± 5.97 µg/cm2) compared to the marketed products, as shown in Figure 1.
Conclusion: The three marketed patches evaluated in this study are instructed to be used for 12 hours. However, this study demonstrates that a minute quantity of CBD is delivered from these patches over a 7-day period. Hence, further pharmacological studies are suggested to evaluate if CBD in such small amounts can positively affect sleep and wellness.
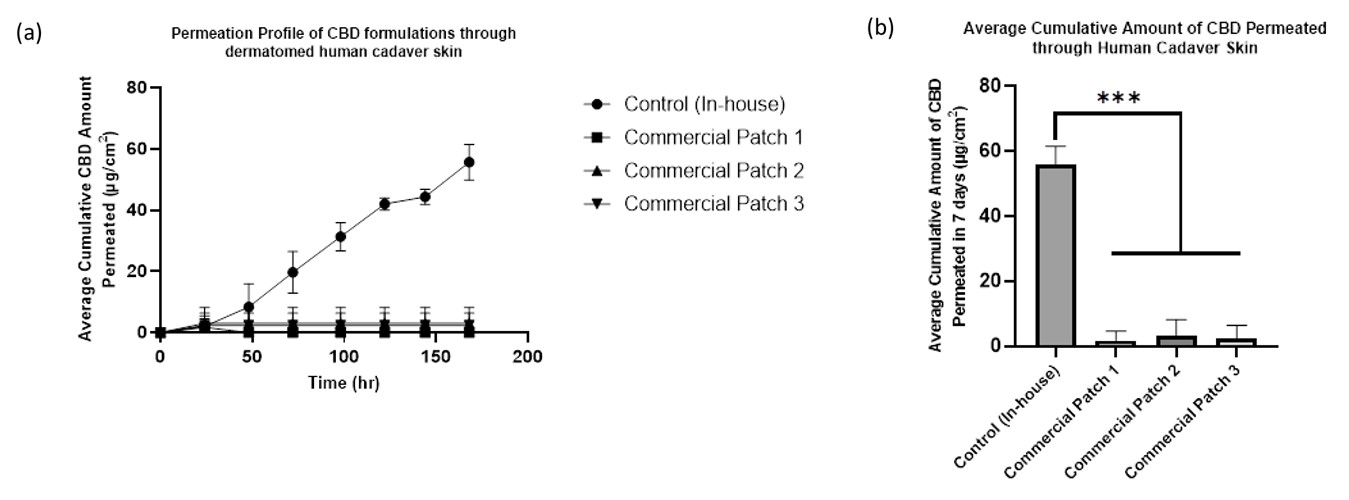 Figure - 1. For control, commercial patch 1, commercial patch 2, and commercial patch 3, (a) Permeation profile of CBD through dermatomed human cadaver skin till 168 hr; (b) Average cumulative amount of CBD permeated through human cadaver skin at the end of 168 hr; All data were represented as Mean ± Standard Deviation; (One-way ANOVA, Brown-Forsythe and welch: *** indicates p ≤ 0.001; n = 3)
Figure - 1. For control, commercial patch 1, commercial patch 2, and commercial patch 3, (a) Permeation profile of CBD through dermatomed human cadaver skin till 168 hr; (b) Average cumulative amount of CBD permeated through human cadaver skin at the end of 168 hr; All data were represented as Mean ± Standard Deviation; (One-way ANOVA, Brown-Forsythe and welch: *** indicates p ≤ 0.001; n = 3)