Formulation and Delivery - Chemical
Category: Poster Abstract
(T1030-11-74) MRI Imaging of SLS 3D Printed Tablets in the Gastrointestinal Tract of Humans
- IS
Iria Seoane-Viano, Ph.D.
University College London
London, England, United Kingdom - IS
Iria Seoane-Viano, Ph.D.
University College London
London, England, United Kingdom - JO
Jun Jie Ong, B.S. (he/him/his)
University College London
LONDON, England, United Kingdom - TP
Tania Pérez-Ramos, M.D.
University Hospital Lucus Augusti
LUGO, Galicia, Spain - EG
Elena Guerra-Baamonde, M.D.
University Hospital Lucus Augusti
LUGO, Galicia, Spain - JL
Jiaqi Liu, M.S. (she/her/hers)
PhD Researcher
University College London
LONDON, England, United Kingdom - JG
Jorge Gonzalez-Ramirez, M.D.
University Hospital Lucus Augusti
LUGO, Galicia, Spain - MV
Manuel Vazquez-Caruncho, M.D.
University Hospital Lucus Augusti
LUGO, Galicia, Spain - AG
Alvaro Goyanes, Ph.D.
FabRx Ltd.
London, England, United Kingdom - AB
Abdul Basit, Ph.D.
University College London
London, England, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: 3D printing is a fast‐growing manufacturing approach with the potential to disrupt the pharmaceutical field by enabling personalised and on demand production of medicines. However, little is known about the in vivo behaviour of these printed medicines in humans. Magnetic resonance imaging (MRI) is an advanced imaging technology that provides real-time images of the environment surrounding the dosage form, such as stomach water content and intestinal wall contractions, making it well-suited to also assess in vivo disintegration of oral forms. In this work, 3D printed torus placebo tablets were prepared using selective laser sintering (SLS) 3D printing technology and their in vivo disintegration time in 12 human volunteers was evaluated. Two different formulations were prepared using two different laser scanning speeds and incorporating manganese chloride in their composition to be able to be visualized by MRI. Moreover, in vitro disintegration studies were also carried out using two different setups; the conventional method based on the use of the USP disintegration apparatus, whose results were compared with the results obtained when the disintegration test was performed in Petri dishes. This is the first in vivo study evaluating the disintegration of 3D-printed oral dosage forms (printlets) in humans.
Methods: SLS 3D printing; To prepare 100 g of formulation, 92 g of hydroxypropylcellulose (HPC) and 5 g of manganese chloride tetrahydrate were blended using a mortar and pestle. 3 g of Candurin® Gold Sheen was added to the formulations to aid printability. Powder mixtures were then transferred to a Desktop SLS printer to fabricate the placebo oral dosage formulations. 3D models of torus printlets (11.5 mm diameter × 5.5 mm height) were exported as a stereolithography (.stl) file into the 3D printer. The surface printing temperature and the chamber temperature were set at 105 °C and 85 ºC, respectively. Two different laser speeds (90 mm/s (SLS90) and 130 mm/s (SLS130) were selected to obtain two different types of formulations; one with a faster disintegration (SLS130) and the other with a longer disintegration time (SLS90). Six printlets of each formulation were printed at the same time. In vitro disintegration study; The in vitro disintegration (n = 3) was tested in 900 mL of 0.1 M hydrochloric acid at 37 ± 0.5 ºC using the USP disintegration apparatus. An additional disintegration test was carried out in Petri dishes containing 50 mL of 0.1 M hydrochloric acid at 37 ± 0.5 ºC. Each torus (n=3) was placed in the centre of the Petri dish and disintegration was regarded as achieved when the printlets lost their form and the components separated. MRI acquisition and in vivo study design; The investigations were performed using a SIGNA explorer with a field strength of 1.5 Tesla at the Radiology Department of the University Hospital Lucus Augusti (HULA). Each of the 12 volunteers took the two types of placebo formulations on two different days at least 7 days apart. The study comprised the following steps. Firstly, the participants were asked to drink 250 mL of water just before entering the MRI machine. Volunteers underwent an initial sequence of axial, coronal, and sagittal location scanning. Subsequently, they were asked to take the formulation with 250 mL of still water. The acquisitions were repeated every 90 seconds until the formulation disappeared.
Results: The printlets showed good uniformity in shape and size (Figure 1). The SLS90 formulation showed a higher mean weight (167.4 ± 0.013 mg compared to 115.7 ± 0.009 mg), possibly due to its lower porosity structure. The mean in vitro and in vivo disintegration times are shown in Table 1. In vivo disintegration times were established based on when the torus loses its shape and leaves a dark trail on the MRI image. For the SLS90 formulation, the mean reported disintegration times were 17.95 ± 1.53 min, whereas for the SLS130 formulation were 12.72 ± 3.41 min (Table 1, Figure 2). The mean disintegration times using the USP apparatus were 7.16 ± 1.04 min and 2.83 ± 0.76 min for the SLS90 and SLS130 formulations, respectively. The results of the study showed that with the modified disintegration tests using Petri dishes, the obtained disintegration times (25.5 ± 4.09 and 18.83 ± 1.89 min for the SLS90 and SLS130 formulations, respectively) were closer to those observed in vivo.
Conclusion: This is the first in vivo study evaluating the disintegration times of 3D-printed oral dosage forms in humans. In particular, using SLS 3D printing and changing laser scanning speeds, two formulations with different disintegration rates were obtained. This was confirmed both in vivo in human volunteers and in vitro. However, the disintegration times obtained in vitro using the USP disintegration apparatus did not correlate with those obtained in vivo. On the other hand, disintegration studies performed on Petri dishes showed disintegration times closer to those obtained in vivo.
Acknowledgements: I.S.-V. acknowledges Consellería de Cultura, Educación e Universidade for her Postdoctoral Fellowship (Xunta de Galicia, Spain; ED481B-2021-019)
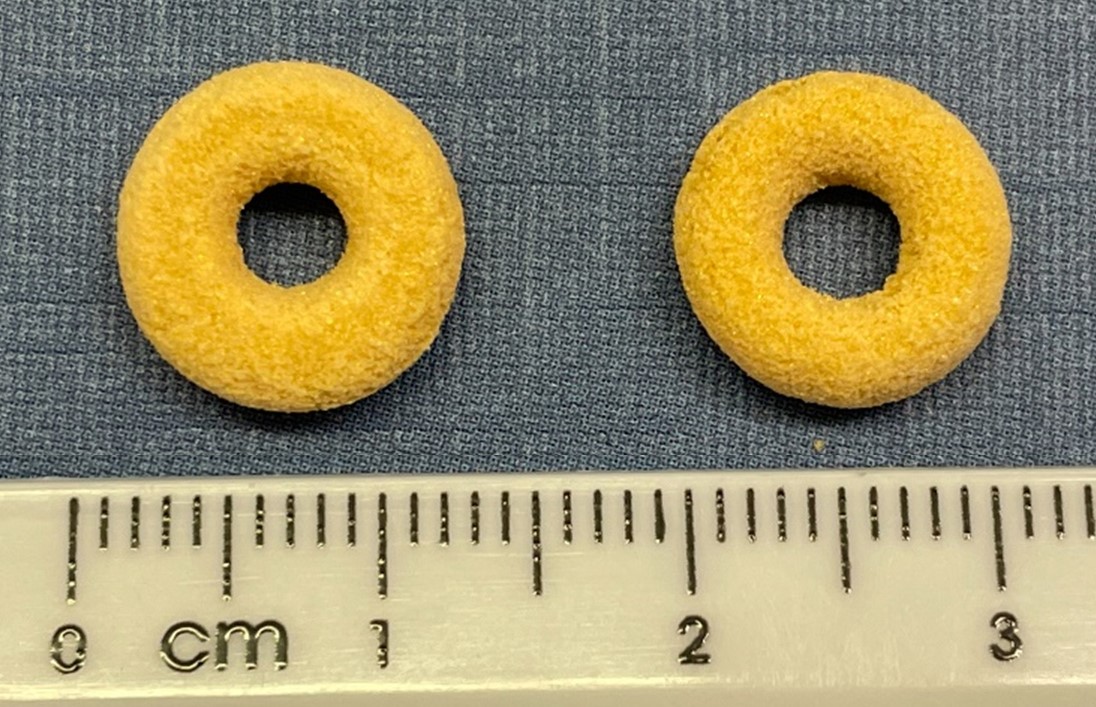 Figure 1. On the left SLS90 and on the right SLS130.
Figure 1. On the left SLS90 and on the right SLS130..jpg) Table 1. In vivo and in vivo disintegration times.
Table 1. In vivo and in vivo disintegration times.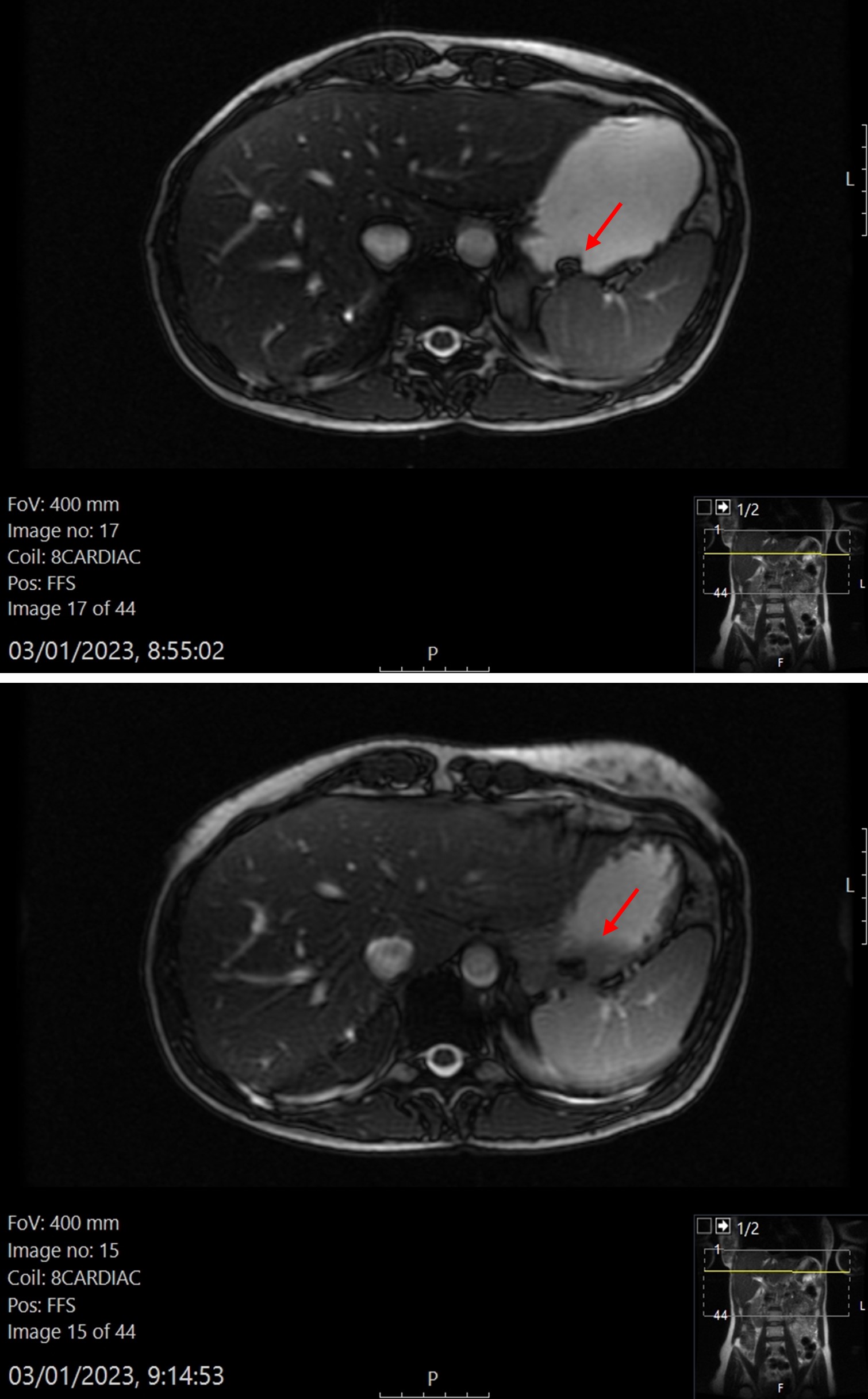 Figure 2. Representative MRI images of the SLS90 formulation. At the top, image taken after ingestion of the formulation. The torus shape of the printlet can be appreciated. At bottom, image taken after the disintegration of the printlet began. A black trail as a result of tablet disintegration can be seen on the image.
Figure 2. Representative MRI images of the SLS90 formulation. At the top, image taken after ingestion of the formulation. The torus shape of the printlet can be appreciated. At bottom, image taken after the disintegration of the printlet began. A black trail as a result of tablet disintegration can be seen on the image.