Formulation and Delivery - Chemical
Category: Poster Abstract
(M1330-01-02) Drug Crystal Growth Rate Decreases in the Presence of Coexistent Drug-Polymer Halogen and Hydrogen Bonds
Monday, October 23, 2023
1:30 PM - 2:30 PM ET

Mustafa Bookwala, MS (he/him/his)
Ph.D. Candidate
Duquesne University
Pittsburgh, Pennsylvania, United States
Mustafa Bookwala, MS (he/him/his)
Ph.D. Candidate
Duquesne University
Pittsburgh, Pennsylvania, United States- IB
Ira S. Buckner, Ph.D. (he/him/his)
Duquesne University
Pittsburgh, Pennsylvania, United States - PW
Peter L.D. Wildfong, Ph.D. (he/him/his)
Duquesne University
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Poorly water-soluble drugs can potentially be formulated as amorphous solid dispersions (ASD), allowing improvement in drug apparent solubility. In this research, formation of different interaction landscapes between drug and polymer was investigated for their impact on the crystal growth step during drug recrystallization. The growth of chlorpropamide, which forms coexistent halogen (X-bonds) and hydrogen bonds (H-bonds) with polyvinylpyrrolidone vinylacetate co-polymer (PVPVA) was compared to that of tolbutamide, a structural analogue that can only form H-bonds with PVPVA. As a control, the crystal growth rates were also determined in polyvinyl acetate (PVAc), a polymer that forms similar interaction landscapes with both drug molecules.
Methods: One-dimensional 1H and 13C solution NMR was performed at ambient conditions using a 500 MHz spectrometer, where deuterated chloroform (CDCl3) was used as the solvent. For sufficient signal-to-noise, 32 scans were collected for 1H, and 1024 scans were collected for 13C nuclei. Chemical shifts were reported relative to TMS for 1H NMR and referenced relative to the CDCl3 for 13C NMR. Drug-polymer mixtures at ratios of 95:5, 90:10, and 85:15 % w/w loading were cryomilled for 60 min. 3-5 mg of milled samples were melt-quenched between glass coverslips to make a film, which was maintained isothermally using a hot-stage. A polarized light microscope (PLM) with 10× or 50× objective and 10× eyepiece was used to observe and measure crystal growth with respect to time, where each reported rate is averaged from 15-25 measurements in 3-5 replicates. Crystal growth rates were measured at 15 °C above the respective glass transition temperatures (Tg) for each drug (Tg,chlorpropamide = 17 oC; Tg,tolbutamide = 6 oC) by tracking the advancement of a growth front in the presence of varying proportions of crystallization inhibitors.
Results: Of the two proton donors in both drug molecules, NMR data showed that the one adjacent to the sulfonyl group had the strongest H-bonding with PVPVA and very similar magnitudes of chemical shifts with increasing PVPVA concentration (Figure 1A). The carbon adjacent to the chlorine atom in the chlorpropamide structure showed clear downfield chemical shifts with increasing PVPVA concentration (Figure 1B), indicative of X-bonding between chlorpropamide and PVPVA. For tolbutamide, the carbon adjacent to the methyl group showed minor shifts, supporting its inability to participate in strong NCI with PVPVA. Comparisons with PVAc revealed minor shifts for the proton donors (Figure 1A) and the carbon adjacent to the X-bond donor (Figure 1B), where the magnitude of the NMR chemical shifts was closer to the scale of instrument variability. These data confirmed that chlorpropamide and tolbutamide formed very weak and similar interaction landscapes with PVAc. Crystal growth rates were exponentially inhibited for both drugs with the addition of increasing quantities of PVPVA. The change in crystal growth rate as a function of PVPVA concentration for chlorpropamide was, however, much higher (Figure 2A). Statistical comparison of slopes (p = 0.00018) indicated significant differences in their values. When PVAc was used to inhibit crystallization, the crystal growth rates slowed down with increasing polymer, but to a smaller extent relative to similar increases in PVPVA. The difference in slopes for the log (crystal growth rate) vs. PVAc concentration was not statistically significant (p = 0.253) (Figure 2B), possibly owing to the potential that similar interaction landscapes form between either analogue and the polymer.
Conclusion: The strength of the drug-polymer interaction landscape can influence the physical stability of ASDs. This work systematically compared two structurally-related API whose strength of interactions with PVPVA differed. Owing to coexistent X- and H-bonding between chlorpropamide and PVPVA, the change in crystal growth rate with respect to PVPVA concentration was significantly different from tolbutamide which could only form H-bonds with the co-polymer. In contrast, PVAc, which formed similar interaction landscapes with both molecules showed no difference in its inhibition of crystal growth.
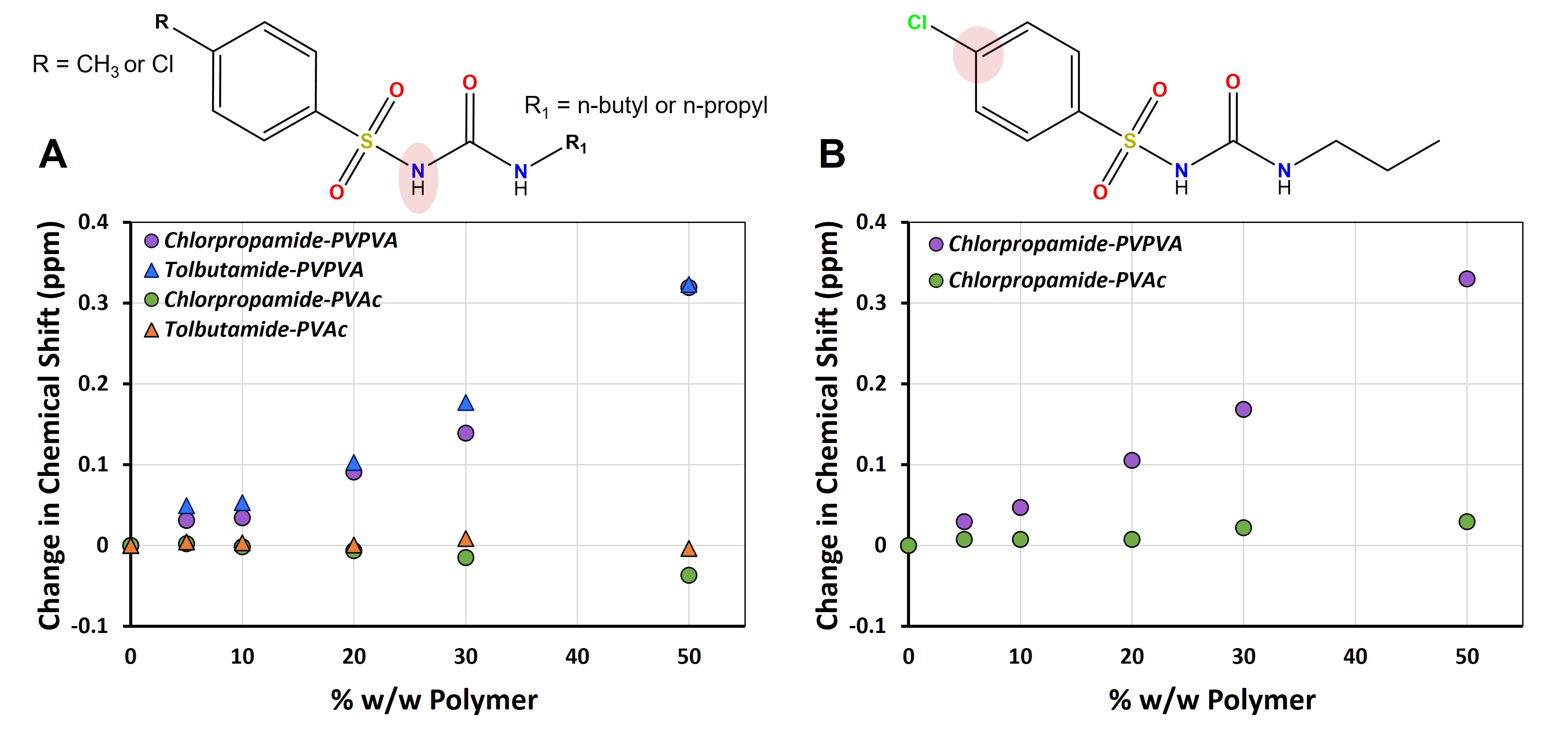 Figure 1: Changes in the magnitude of chemical shift are shown for A) the proton donor adjacent to the sulfonyl group for chlorpropamide and tolbutamide with increasing PVPVA or PVAc concentration and for B) the carbon adjacent to the halogen bond donor Cl in chlorpropamide with increasing PVPVA and PVAc content.
Figure 1: Changes in the magnitude of chemical shift are shown for A) the proton donor adjacent to the sulfonyl group for chlorpropamide and tolbutamide with increasing PVPVA or PVAc concentration and for B) the carbon adjacent to the halogen bond donor Cl in chlorpropamide with increasing PVPVA and PVAc content. 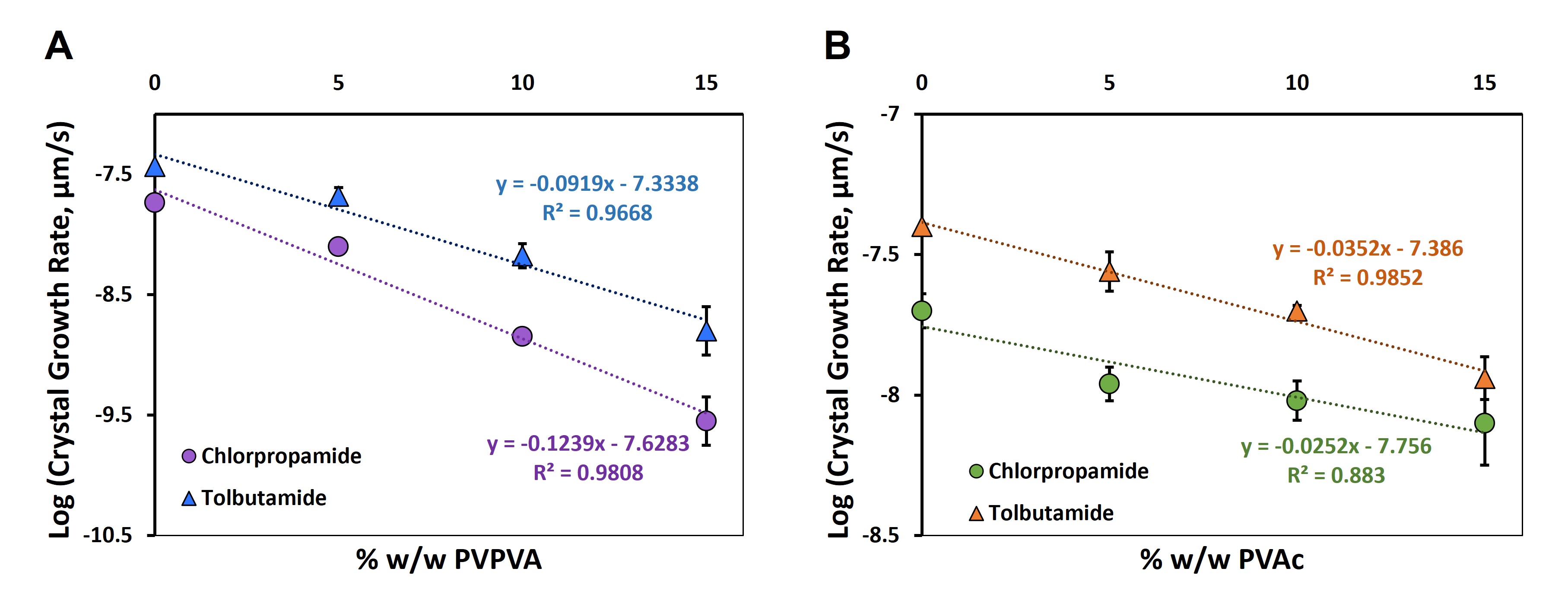 Figure 2: Crystal growth rates were measured for chlorpropamide and tolbutamide in the presence of A) PVPVA or B) PVAc crystallization inhibitors. As the drugs interacted with PVPVA to different extents, the slopes of log of crystal growth rate vs. PVPVA concentration were found to be statistically different. However, as the drugs interacted similarly with PVAc, no statistical difference was observed between the slopes.
Figure 2: Crystal growth rates were measured for chlorpropamide and tolbutamide in the presence of A) PVPVA or B) PVAc crystallization inhibitors. As the drugs interacted with PVPVA to different extents, the slopes of log of crystal growth rate vs. PVPVA concentration were found to be statistically different. However, as the drugs interacted similarly with PVAc, no statistical difference was observed between the slopes.