Formulation and Delivery - Biomolecular
Category: Poster Abstract
(M0930-02-08) A Novel Immunization Strategy against Malaria Using Microneedles and Virus-Like Particles Vaccine
- AL
Aidan Leyba, BS
University of New Mexico
Albuquerque, New Mexico, United States - PM
Pavan Muttil, Ph.D.
University of New Mexico
Albuquerque, New Mexico, United States - MR
Mohammad Razmjoo, Pharm.D.
University of New Mexico
Albuquerque, New Mexico, United States - AF
Alexandra Francian, Ph.D.
University of New Mexico
Albuquerque, New Mexico, United States - AB
Amelia Bierle, MS
University of New Mexico
Albuquerque, New Mexico, United States - BR
Bryce Roberts, BS
University of New Mexico
Albuquerque, New Mexico, United States - LJ
Lucie Jelinkova, BS
University of New Mexico
ABQ, New Mexico, United States - DM
David McChesney
University of New Mexico
Albuquerque, New Mexico, United States - BC
Bryce Chackerian, Ph.D.
University of New Mexico
ABQ, New Mexico, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The need for novel immunization strategies in underdeveloped nations with warm climates is highlighted by the lack of efficient vaccines against malaria. In conjunction with inefficient malarial vaccines, immunization coverage in these regions is hampered by the lack of robust cold-chain facilities that allows vaccines to be stored and transported to remote areas under extreme temperature and humidity conditions. In addition, malaria-endemic regions have a scarcity of trained healthcare professionals for proper vaccine administration using hypodermic needles; accidental needle-stick injuries are common during mass immunization. This study demonstrates the successful development of two novel malarial vaccine candidates, TRIO and Sialokinin VLPs (Virus-like particles), with polymer-based dissolvable microneedles (MN). VLPs are self-assembling viral structural proteins that mimic the overall structure of a virus but lack the genetic material required for viral replication. TRIO and Sialokinin are salivary proteins derived from the Anopheles and Aedes mosquitos, respectively, and their peptides are expressed on the surface of VLPs. Our formulations serve as two novel vaccine candidates against malaria and have been separately loaded into MNs. Developing MNs containing VLPs will allow for easy vaccine administration while maintaining vaccine stability during storage and transportation.
Methods: Manufacturing our VLP-loaded MNs is a 3-stage process completed within 24 hours (Figure 1). The first stage begins with creating a stock solution of poly(acrylic acid) (PAA) polymer that is gently vortex-mixed with VLPs (conjugated to either TRIO or Sialokinin) to create a homogenous solution. The second stage involves pipetting this solution into polydimethylsiloxane (PDMS) microneedle molds and centrifuging them to allow the polymeric solution to fill the mold wells. The PDMS mold contains an array of 10x10 wells and can produce up to 100 needles per MN patch. Centrifuge parameters include 60 minutes of spinning at 30°C and using a centrifugal force of 1350. The first round of centrifugation accounts for the needle layer of the MNs; a second centrifugation step was performed after pipetting PAA (without VLPs) on top of the first layer and centrifuging with the above parameters to account for the backing layer that provides structural support to the needles. Finally, in stage 3, the MNs are dried in a desiccator for less than 24h. Finally, the MNs are removed from their molds using the back of a marker and double-sided tape. Removing the MNs from their molds at a 90° angle is essential to prevent bending or breaking of the needles. Following the fabrication steps, the dried VLP-loaded MNs are dissolved in phosphate-buffered saline (PBS) to evaluate VLPs-integrity using transmission electron microscopy (TEM) (Figure 2). First, we dissolved a pre-determined number of MNs in PBS to achieve a specified concentration of VLPs and compared them with pure VLPs (no MNs) of the same concentration. Our laboratory previously utilized MNs in a study involving CIS43-VLPs and MF59 adjuvant (Figure 3). The MNs were stored at elevated temperature and humidity conditions for three months and then used to immunize mice to study in vivo immunogenicity. The MNs preparation for this study took multiple days; however, this study proved that MNs loaded with a malarial vaccine were immunogenic in mice.
Results: Microneedles were fabricated using a novel fast-manufacturing method (24 hours, Figure 1). The image shows needle sharpness and appropriate length (~410 microns) important to penetrate mouse skin and to release the VLPs in the epidermis, which is rich in immune cells such as Langerhans cells and dendritic cells. TEM imaging showed that the VLPs did not lose their integrity after loading into the MNs (Figure 2). We also observed minimal VLPs aggregation after dissolving the VLPs from within the MNs. Our preliminary data showed a robust immune response between VLPs delivered using MNs and the intramuscular control group (delivered using hypodermic needles). This indicates that MNs can be loaded with a vaccine and stored in extreme storage conditions (where malaria is endemic) for long periods, providing adequate immunogenicity (Figure 3).
Conclusion: Our research has focused on creating dissolvable PAA-based MNs loaded with a novel malarial vaccine (VLPs based). The fast fabrication process did not compromise the needle sharpness to penetrate the ex vivo skin layers of mice. Further, faster manufacturing methods will allow large-scale vaccine production in case of another pandemic. Future studies will evaluate the stability of our VLPs-containing MNs at high temperatures and humidity to mimic the environmental conditions in malarial-endemic regions. Future animal studies will also compare the immune response of MN injection compared to VLPs delivered by the conventional intramuscular injection.
Acknowledgements:/b> The preclinical studies (preliminary data) described in this poster were supported by the National Institute of General Medical Sciences (3UT2GM130166-02S1) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001449)
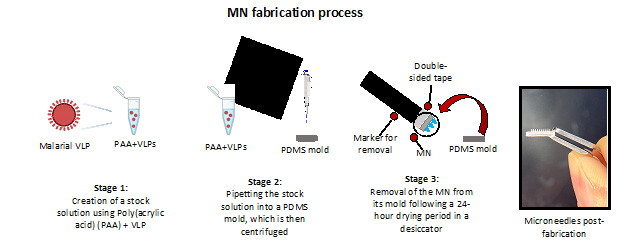 Three-stage MN fabrication process containing VLPs against Malaria
Three-stage MN fabrication process containing VLPs against Malaria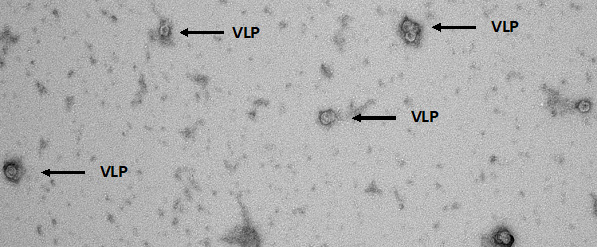 Transmission Electron Microscopy image of VLPs released from MN to show VLPs integrity was maintained during the MN fabrication process
Transmission Electron Microscopy image of VLPs released from MN to show VLPs integrity was maintained during the MN fabrication process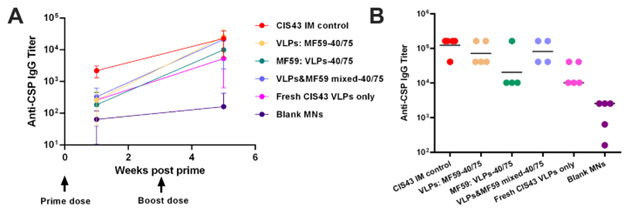 Immune response of stabilized MN with CIS43 VLPs + MF59 adjuvant: The mMNs were stored for three months at 40°C/75% relative humidity (RH). Figures A and B represent antibody titers in vaccinated mice 5 weeks and 11 months after the first vaccine dose, respectively. In the study, we also compared the immunogenicity of stabilized mMNs with control groups, including freshly made mMNs, and vaccines administered intramuscularly and subcutaneously.
Immune response of stabilized MN with CIS43 VLPs + MF59 adjuvant: The mMNs were stored for three months at 40°C/75% relative humidity (RH). Figures A and B represent antibody titers in vaccinated mice 5 weeks and 11 months after the first vaccine dose, respectively. In the study, we also compared the immunogenicity of stabilized mMNs with control groups, including freshly made mMNs, and vaccines administered intramuscularly and subcutaneously.