Formulation and Delivery - Chemical
Category: Poster Abstract
(M1230-11-70) A Nature Inspired Octopus-Shaped 3D Printed Floating Drug Delivery System
Monday, October 23, 2023
12:30 PM - 1:30 PM ET
- VR
Vishvesh Raje, MS (he/him/his)
Ph.D. Student
St. John's University
Queens, New York, United States - VR
Vishvesh Raje, MS (he/him/his)
Ph.D. Student
St. John's University
Queens, New York, United States - SD
Snehal S. Daware, MS (she/her/hers)
St. John's University
Jamaica, New York, United States - KP
Ketan Patel, Ph.D.
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Gastroretentive Drug Delivery Systems (GRDDS) have been widely explored for improving drug absorption and bioavailability with shorter absorption windows and lesser residence time. The complexities of the GRDDS not only require a combination of materials but also demand innovative design approaches to achieve optimal drug delivery performance. 3D printing offers customization of complex geometries, precise drug release, and rapid prototyping for the GRDDS. Current research explores a nature-inspired design, octopus-shaped 3D printed tablets – “Octoprints”, by combining the benefits of material properties and customizability of the fused deposition modeling (FDM) 3D printing. The octoprint offers advantages such as prolonged residence time and controlled drug release, which could improve the efficacy and safety of medications with a short absorption window.
Methods: Drug-loaded filaments of a model drug - levodopa were prepared using hot melt extrusion (HME) technology with an 11mm twin screw extruder. Three types of material viz. a) Swellable polymers- Poly ethyl oxide (PEO), Hydroxy propyl methyl cellulose (HPMC) b) Polymers for sustained release- Ethyl cellulose (EC), Hydroxy propyl methyl cellulose acetate succinate (HPMC-AS) c) Printability enhancing polymers- Hydroxy propyl cellulose (HPC), Poly(2-ethyl-2-oxazoline) (PETOX) were screened. To assess the processability of complex drug-polymer systems in HME, preliminary rheology studies were conducted using the Discovery Hybrid Rheometer-2 (DRH-2). The solid-state characterization was done using Differential Scanning Calorimetry (DSC) and Powder X-ray Diffraction (PXRD) techniques. Furthermore, the printability of the extruded filaments was evaluated by a three-point bend test (3PBT) using TA.XTplus Texture Analyzer. The octoprints were designed in Tinkercad software. Critical printing parameters such as extruder temperature, printing speed, deposition layer thickness, etc. were optimized for uniform printing. The re-floating ability of octoprint was tested by immersing it in the dissolution media (0.1 N HCl) for 5 seconds per hour. In vitro drug release was performed in 250 mL of 0.1N HCl (pH 1.2).
Results: The HME was conducted within the temperature range of 120-180oC. In order to achieve sustained release of the water-soluble drug, levodopa; it was necessary to use EC / HPMAS and PEO. However, filaments containing more than 15% w/w of EC or HPMCAS had sufficient strength to pass the 3PBT but were not suitable for 3D printing due to poor adhesion between the deposition layers. This caused flimsiness and inconsistencies in the structure of the octoprint. Additionally, filaments containing more than 30% w/w of PEO resulted in poor mechanical properties. Therefore, to improve the filament printability a composition was selected with more than 50% w/w of the printable polymer HPC, less than 15% w/w of EC, and less than 30% w/w of PEO. The balance and buoyancy of the octoprints were influenced by the weight distribution between the head part and leg part. Hence, changing the infill densities of the octoprint head (0%, 20%, 50%, and 95%) resulted in variations in its weight, which consequently affected the center of gravity and floating behavior. The design of the octoprint was optimized to ensure that the center of gravity was positioned below the head part, but slightly above half of the leg's length, to maintain a vertical position while floating for every infill density.
The dimensions of octoprints were 8mm x 8mm x 15mm, which fit within a size 000 capsule. Octoprints exhibited sustained release of levodopa over 8hrs. Primarily, it was expected that the octoprints with 10% w/w and 20% w/w of PEO would have a different drug release. However, the drug release was not significantly affected by the PEO concentration. To elucidate this, release kinetics was conducted using various models. The best fit was for the Higuchi model with an R2 value of 0.91, which suggests that the drug release is diffusion controlled. The head part of the octoprints was floating and intact till the end of the dissolution. However, the leg part was comparatively weak and started dissolving starting from the first 15mins of the dissolution. The octoprints showed substantial swelling where the height of the octopus increased more than 200%.
Conclusion: The nature-inspired design of the octopus-shaped drug delivery system (octoprints) using fused deposition modeling (FDM) 3D printing shows promising results for GRDDS. The use of an appropriate combination of polymers and design helped to achieve desirable mechanical properties and floating behavior of the octoprints.
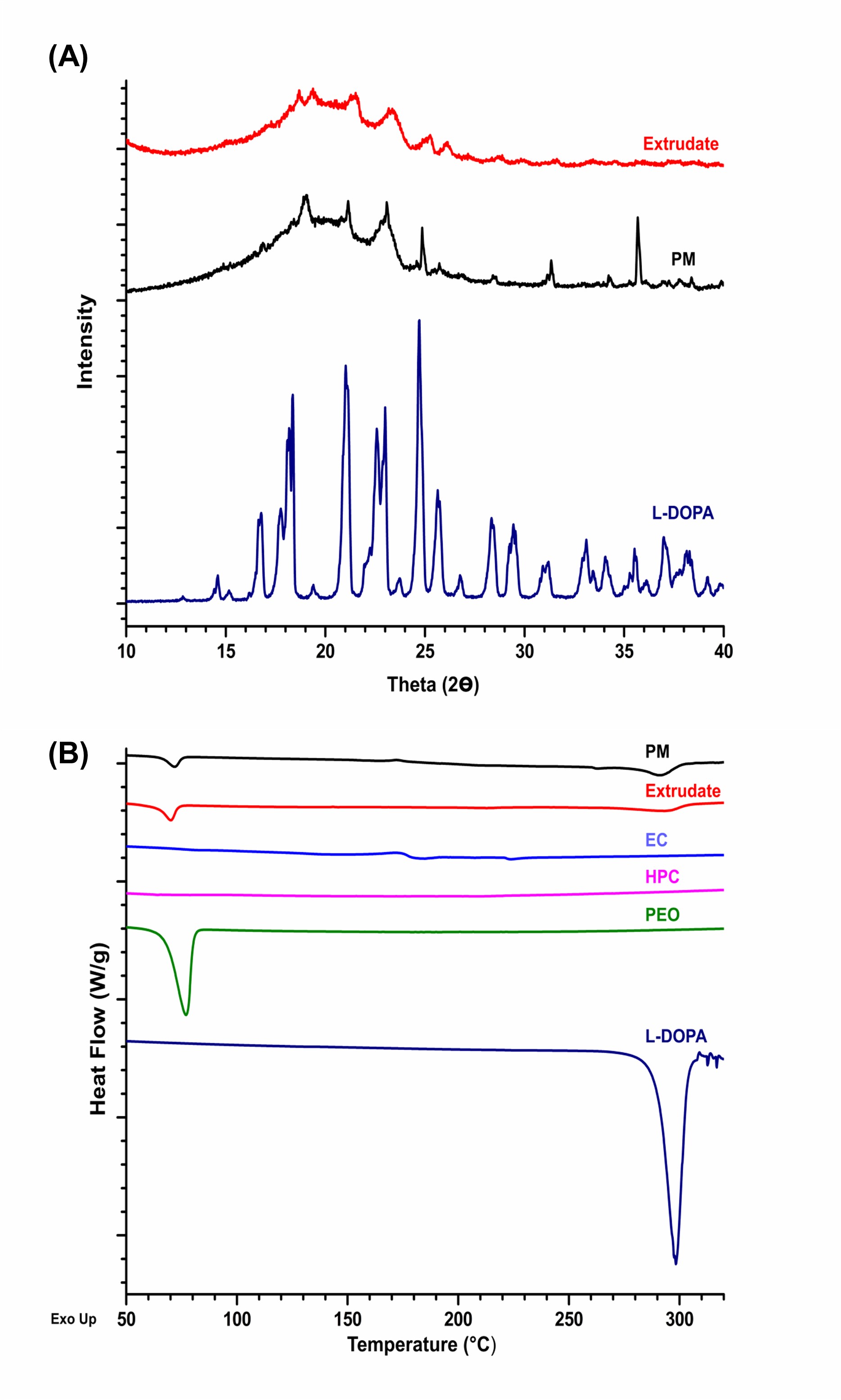 Fig. 1. Solid state characterization. (A) PXRD analysis for the pure drug- levodopa (L-Dopa), powdered mixtures (PM), and the extrudate. A broad halo for the extrudates was observed. (B) DSC analysis for pure drug- levodopa (L-Dopa), polymers, powdered mixtures of final formulation (PM), and the extrudate. L-Dopa showed a sharp melting point of 300 Degree Celsius. The extrudate showed an absence of characteristic endothermic peak for L-DOPA. This result suggests the molecular dispersion of the drug in extrudates.
Fig. 1. Solid state characterization. (A) PXRD analysis for the pure drug- levodopa (L-Dopa), powdered mixtures (PM), and the extrudate. A broad halo for the extrudates was observed. (B) DSC analysis for pure drug- levodopa (L-Dopa), polymers, powdered mixtures of final formulation (PM), and the extrudate. L-Dopa showed a sharp melting point of 300 Degree Celsius. The extrudate showed an absence of characteristic endothermic peak for L-DOPA. This result suggests the molecular dispersion of the drug in extrudates. 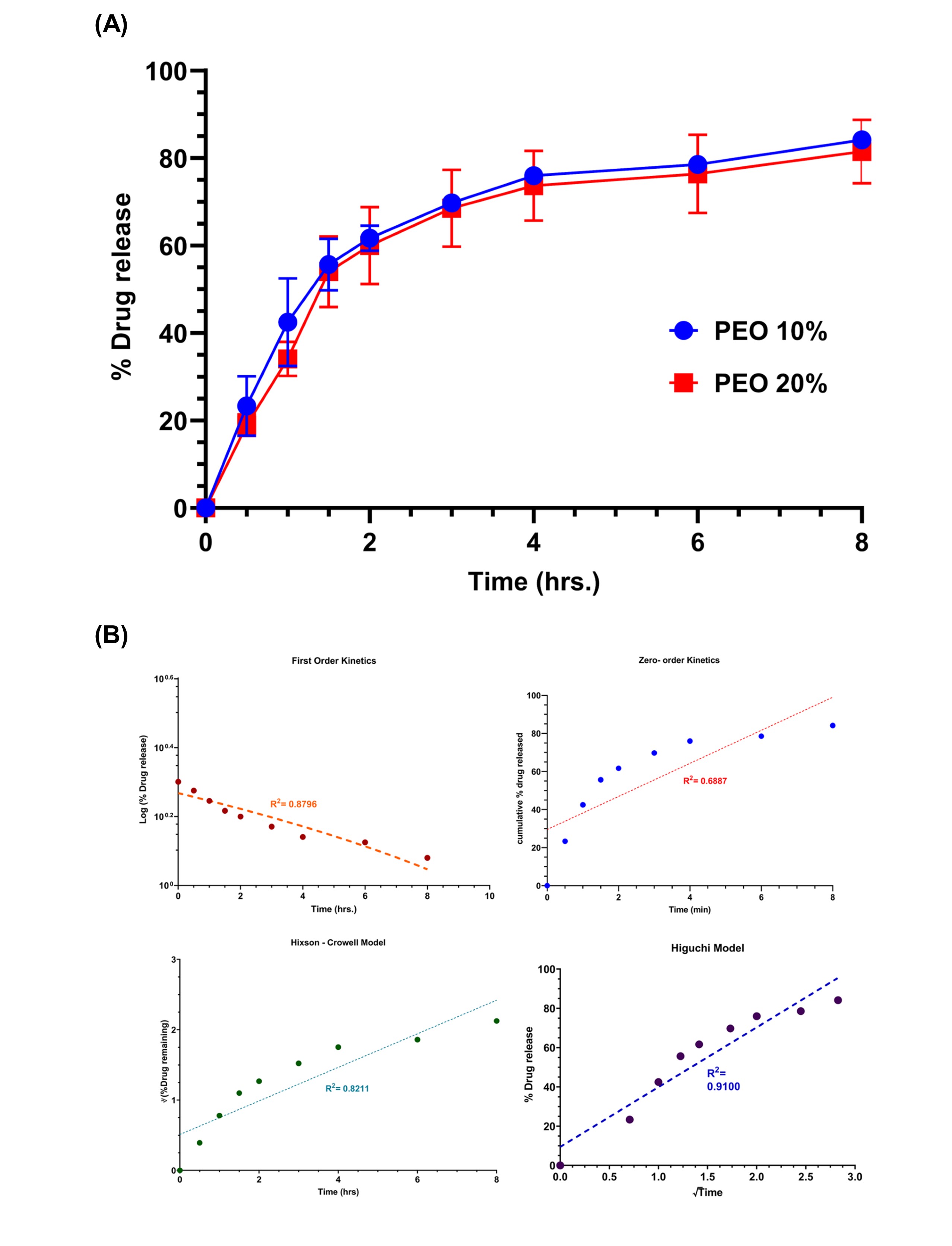 Fig. 2. In vitro release of octoprints in 250mL of 0.1N HCl. (A) A sustained release of the L-DOPA (water-soluble drug) was observed for more than 8hrs. The %w/w of PEO in the extrudates did not affect the drug release significantly. (B) Drug release kinetics for different models. Higuchi model showed the best fit suggesting diffusion-controlled drug release.
Fig. 2. In vitro release of octoprints in 250mL of 0.1N HCl. (A) A sustained release of the L-DOPA (water-soluble drug) was observed for more than 8hrs. The %w/w of PEO in the extrudates did not affect the drug release significantly. (B) Drug release kinetics for different models. Higuchi model showed the best fit suggesting diffusion-controlled drug release. 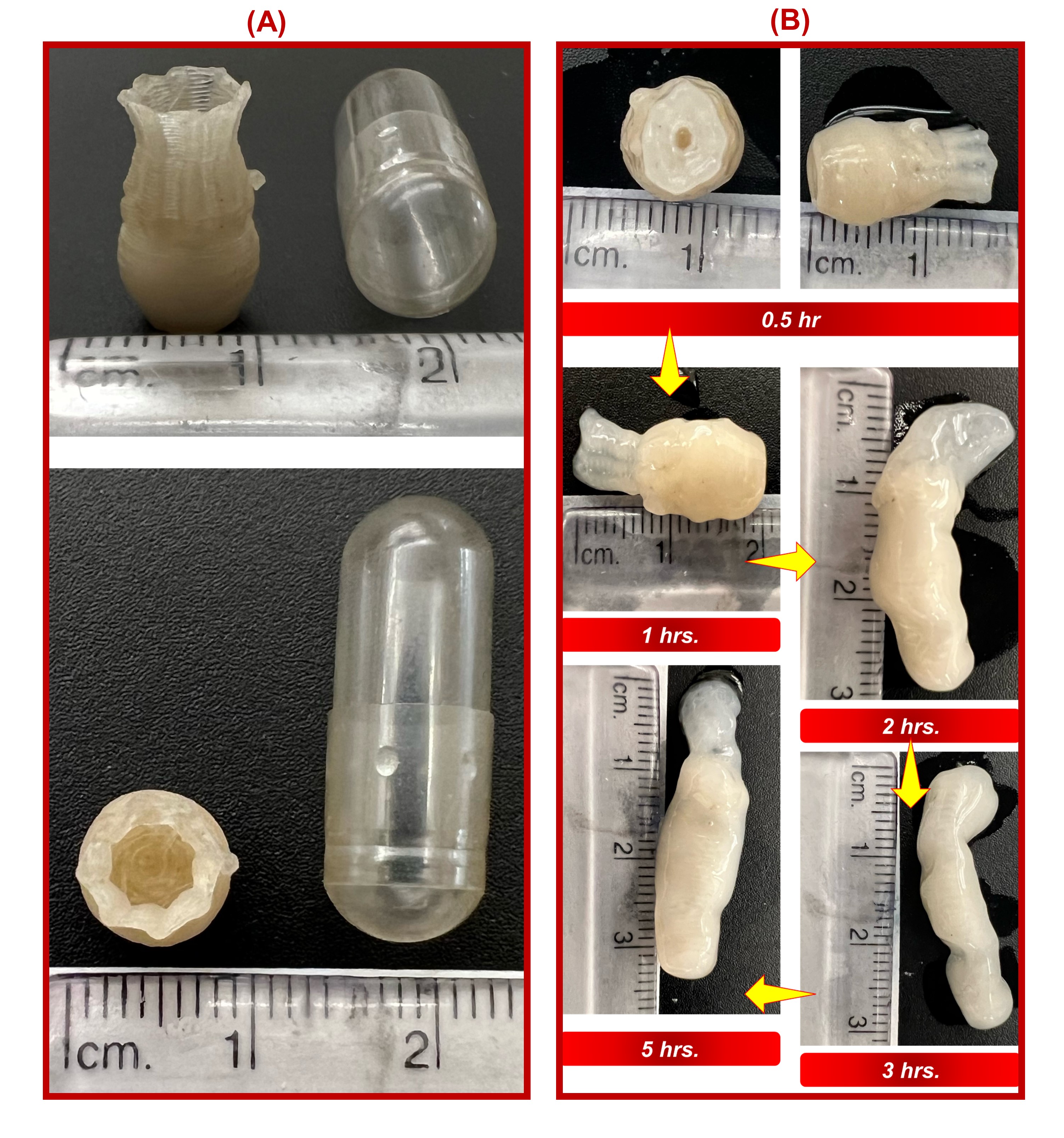 Fig. 3. (A) Size of the octoprints compared to a size 000 capsule (B) Swelling behavior of the octoprints. A significant increase in the height (more than 200% ) for the octoprint was observed after 5hrs during dissolution.
Fig. 3. (A) Size of the octoprints compared to a size 000 capsule (B) Swelling behavior of the octoprints. A significant increase in the height (more than 200% ) for the octoprint was observed after 5hrs during dissolution.