Formulation and Delivery - Chemical
Category: Poster Abstract
(M1330-11-72) Engineering Gefitinib-Loaded Inhalable Solid Lipid Nanoparticles Using an Organic Solvent-Free Homogenization Method for the Treatment of Non-small Cell Lung Cancer
Monday, October 23, 2023
1:30 PM - 2:30 PM ET
- VP
Vasudha Prithipaul, B.S. (she/her/hers)
St. John's University
Queens, New York, United States - VP
Vasudha Prithipaul, B.S. (she/her/hers)
St. John's University
Queens, New York, United States - DB
Druva Sarika Barji, Ph.D. (she/her/hers)
St. John's University
Malvern, Pennsylvania, United States - NK
Nitesh Kunda, Ph.D.
St. John's University
New York, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Gefitinib is a reversible competitive tyrosine kinase inhibitor (TKI), that targets the epidermal growth factor receptor (EGFR) Exon 19 deletion or exon 21 (L858R) substitution mutation, belonging to the first-generation of EGFR TKIs. Gefitinib has been approved by the FDA to treat advanced non-small cell lung cancer (NSCLC) in 2003 and is currently being administered orally or through the naso-gastric route. Several side-effects are associated with the oral administration of gefitinib including liver inflammation and perforation of the stomach and intestines. Based on previous literature, it is hypothesized that encapsulating gefitinib in a solid lipid matrix would aid in the reduction of its side effects. Further, changing the route of administration from the oral route to the pulmonary route would aid in avoiding the first-pass effect, reduce the dose administered, and minimize systemic side-effects. The goal of this project is to engineer an inhalable solid lipid nanoparticle loaded with gefitinib (GEFSLNs) for the treatment of NSCLC to administer a lower dose of the drug and a significant reduction in its systemic side-effects.
Methods: The GEFSLNs, made using GeleolTM and Tween 80 using a solvent-free homogenization method, were evaluated for particle size, zeta potential, and entrapment efficiency (EE). The crystallinity and encapsulation of the formulation was evaluated by Differential Scanning Calorimetry (DSC), Powder X-ray Diffraction (PXRD), and Fourier Transform Infrared Spectroscopy (FTIR). In addition, the nebulization efficiency of SLNs and lung deposition profile was investigated using the Next Generation Impactor (NGI) operated at 15 L/min using a PARI LC nebulizer. The drug release studies were conducted in PBS, with a pH adjusted to 7.4, at 37 °C. In vitro cell studies were conducted on the NSCLC cell line HCC827, which bears a mutation in the EGFR tyrosine kinase domain causing the E746-A750 deletion. The cell viability data was obtained by performing the MTT assay, which is a colorimetric assay that assesses cell metabolic activity, for 72 h. The IC50 value on this specific cell line was determined and the concentration obtained was used to treat the cells in further experiments. A cellular uptake study was also conducted with the SLN formulation encapsulating nile red, a hydrophobic fluorescent dye, prepared utilizing the same technique as that of the GEFSLNs. In addition, a colony formation assay was carried out where cells were treated with the free drug as well as the formulation and the colony forming activity of the cells were gauged and compared to a control.
Results: The GEFSLNs displayed a mean particle size of 68.06 ± 0.54 nm, a zeta potential of -25.17 ± 0.67 mV, and an encapsulation efficiency of 65.5 ± 4.7% (Fig.1A). Figure 1B illustrates the FTIR spectra, it is apparent that the molecular fingerprint of gefitinib is absent on GEFSLNs’ FTIR spectrum, confirming the successful removal of the free drug from the formulation and the total encapsulation of the drug inside the nanoparticle. The DSC thermogram of the drug showed a melting peak at 195°C while in that of GEFSLN, the peak disappeared demonstrating that the encapsulated drug was in the amorphous state (Fig. 1C). The XRD analysis confirmed absence of crystalline peaks corresponding to the free drug indicating encapsulation of the drug in the lipid matrix (Fig. 1D). After solid-state characterization, an in vitro aerosolization study was conducted where the nebulized microdroplet of GEFSLNs revealed a mass median aerodynamic diameter of 4.79 ± 0.12 μm and a fine particle fraction of 69.5 ± 0.36% corroborating its deep lung deposition capability as determined by the NGI study (Fig. 2B). Further, the drug release study indicated that the formulation exhibited a sustained release, displaying no burst release, with an average of 42.9 ± 0.48% released over 72 hours (Fig. 2A). The IC50 value of the free drug on HCC827 was 15.08 ± 0.06 nM, as compared to that of the GEFSLNs which was 8.77 ± 1.31nM, demonstrating an approximate 2-fold reduction in IC50 (Fig 2D). The cell uptake study revealed that within 2 hours, the nanoparticle formulation was up taken by the cells and were found settling around the nucleus (stained with DAPI) of the cell. Figure 3 shows the clonogenic assay data where it is evident that the wells treated with the formulation at 8 nM inhibited the formation of colonies. The average number of colonies formed in wells treated by the formulation at IC50 concentration was 10 as compared to 64 colonies formed in the control wells.
Conclusion: The solid lipid nanoparticles encapsulated with gefitinib offer promising potential as an inhalable formulation with efficient lung deposition for the treatment of NSCLC. The efficacy of the drug significantly increased in vitro when encapsulated and this can potentially aid in reducing the dose of the drug. However, further studies are required to evaluate the effects of the formulation in vivo.
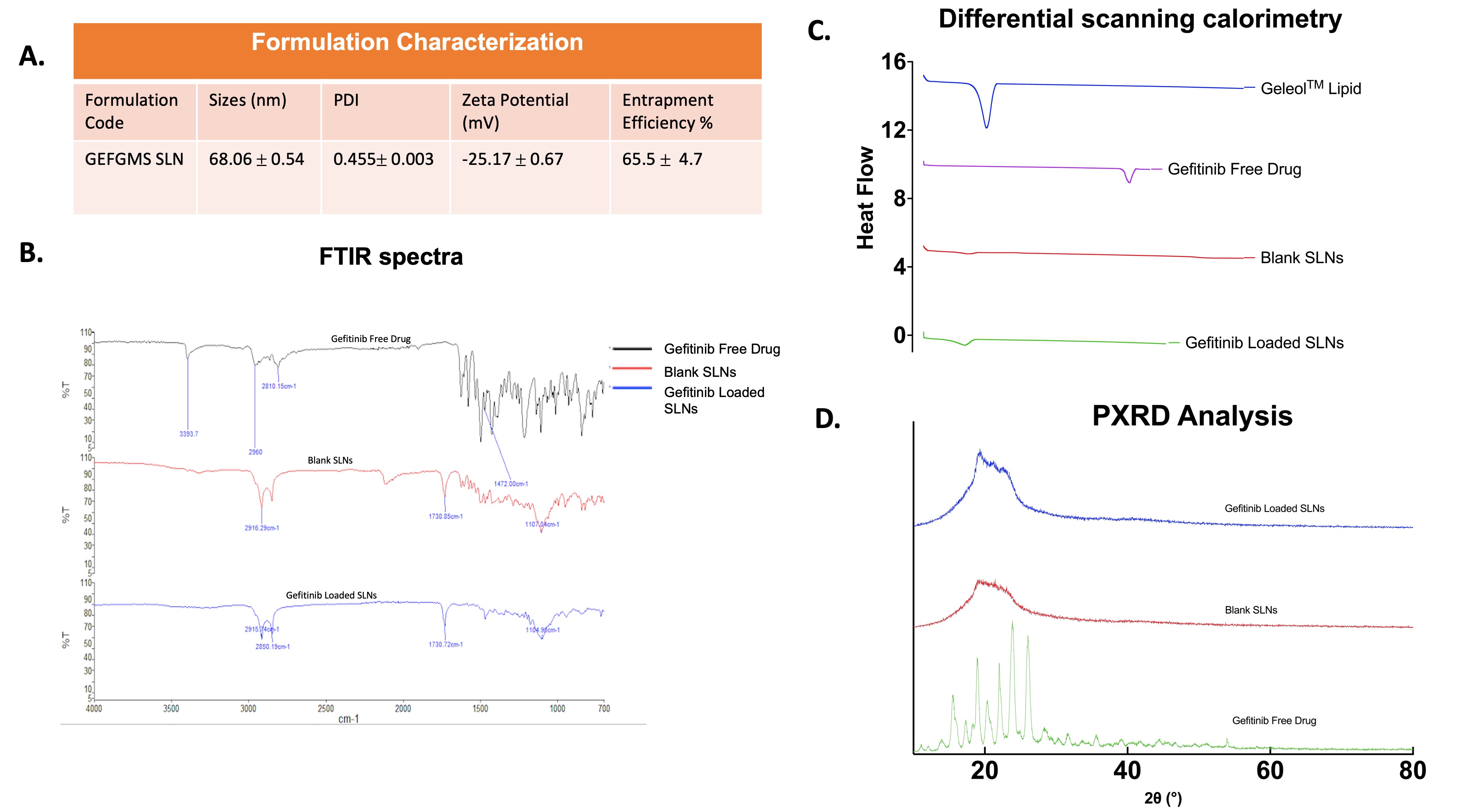 Figure 1: A) Table 1: Particle size, PDI, zeta potential, and entrapment efficiency of gefitinib-loaded SLNs, (n = 3, Mean ± SD), B) FTIR spectra of the free drug, the blank formulation, and the drug loaded formulation, C) Thermograms of the lipid, the free drug, the blank nanoparticles, and the drug loaded nanoparticles, D) X-ray diffractogram of the free drug, the blank formulation, and the drug loaded formulation.
Figure 1: A) Table 1: Particle size, PDI, zeta potential, and entrapment efficiency of gefitinib-loaded SLNs, (n = 3, Mean ± SD), B) FTIR spectra of the free drug, the blank formulation, and the drug loaded formulation, C) Thermograms of the lipid, the free drug, the blank nanoparticles, and the drug loaded nanoparticles, D) X-ray diffractogram of the free drug, the blank formulation, and the drug loaded formulation.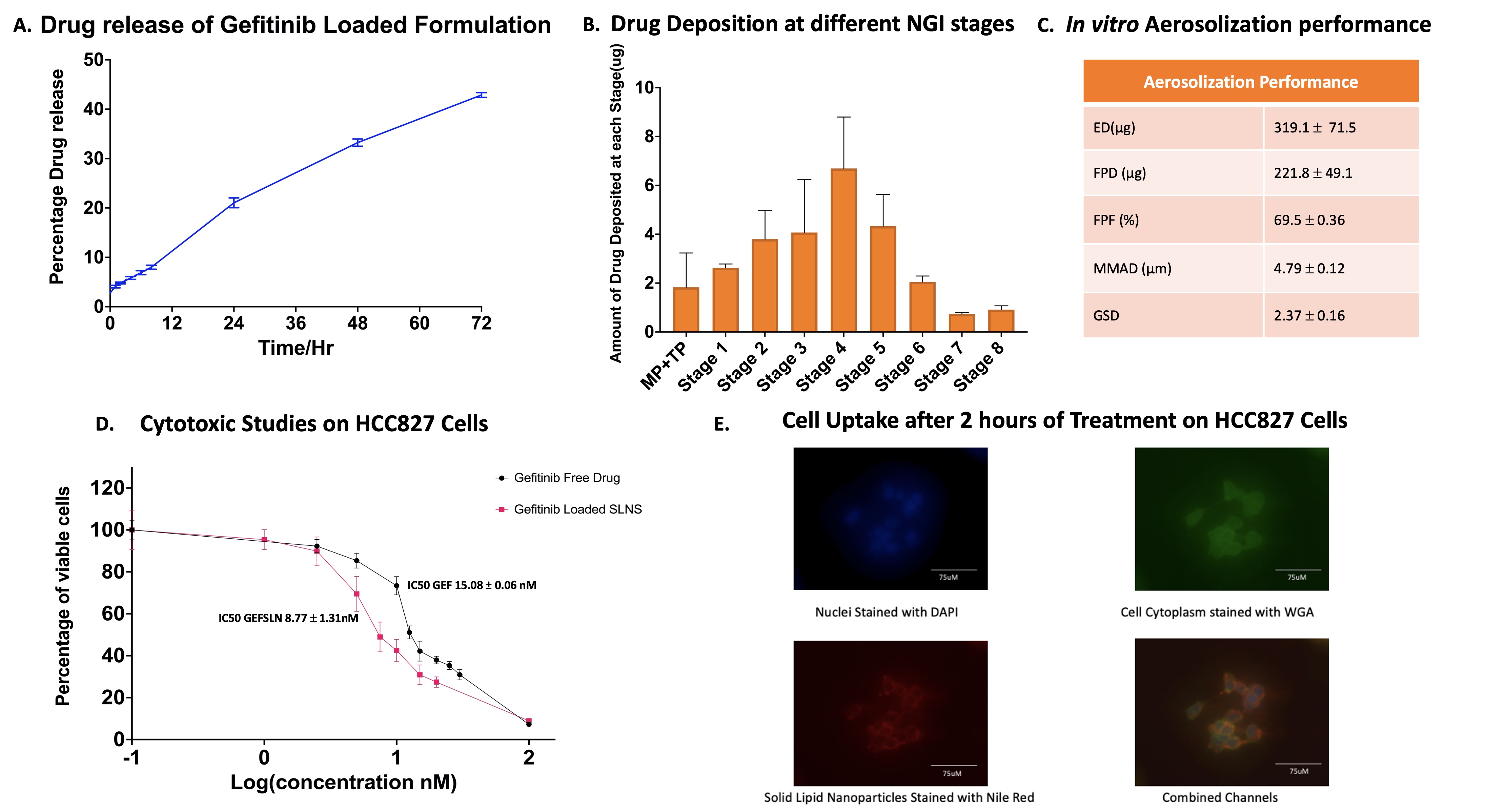 Figure 2: A) Drug release at pH 7.4n PBS, (n = 3, Mean ± SD), B) Chart representing the amount of drug deposited at each stage after nebulization (n = 3, Mean ± SD), C) Aerosolization performance of the nebulized formulation including the emitted dose, fine particle fraction, mass median aerodynamic diameter, and geometric standard deviation, D) Cytotoxic Studies of gefitinib and GEFSLNs on HCC827, (n = 3, Mean ± SD), E) Fluorescent images of the cellular uptake of the formulation loaded with Nile Red in HCC827 with 4′,6-diamidino-2-phenylindole (DAPI) staining the nucleus blue and Wheat germ agglutinin (WGA) staining the cell cytoplasm green.
Figure 2: A) Drug release at pH 7.4n PBS, (n = 3, Mean ± SD), B) Chart representing the amount of drug deposited at each stage after nebulization (n = 3, Mean ± SD), C) Aerosolization performance of the nebulized formulation including the emitted dose, fine particle fraction, mass median aerodynamic diameter, and geometric standard deviation, D) Cytotoxic Studies of gefitinib and GEFSLNs on HCC827, (n = 3, Mean ± SD), E) Fluorescent images of the cellular uptake of the formulation loaded with Nile Red in HCC827 with 4′,6-diamidino-2-phenylindole (DAPI) staining the nucleus blue and Wheat germ agglutinin (WGA) staining the cell cytoplasm green.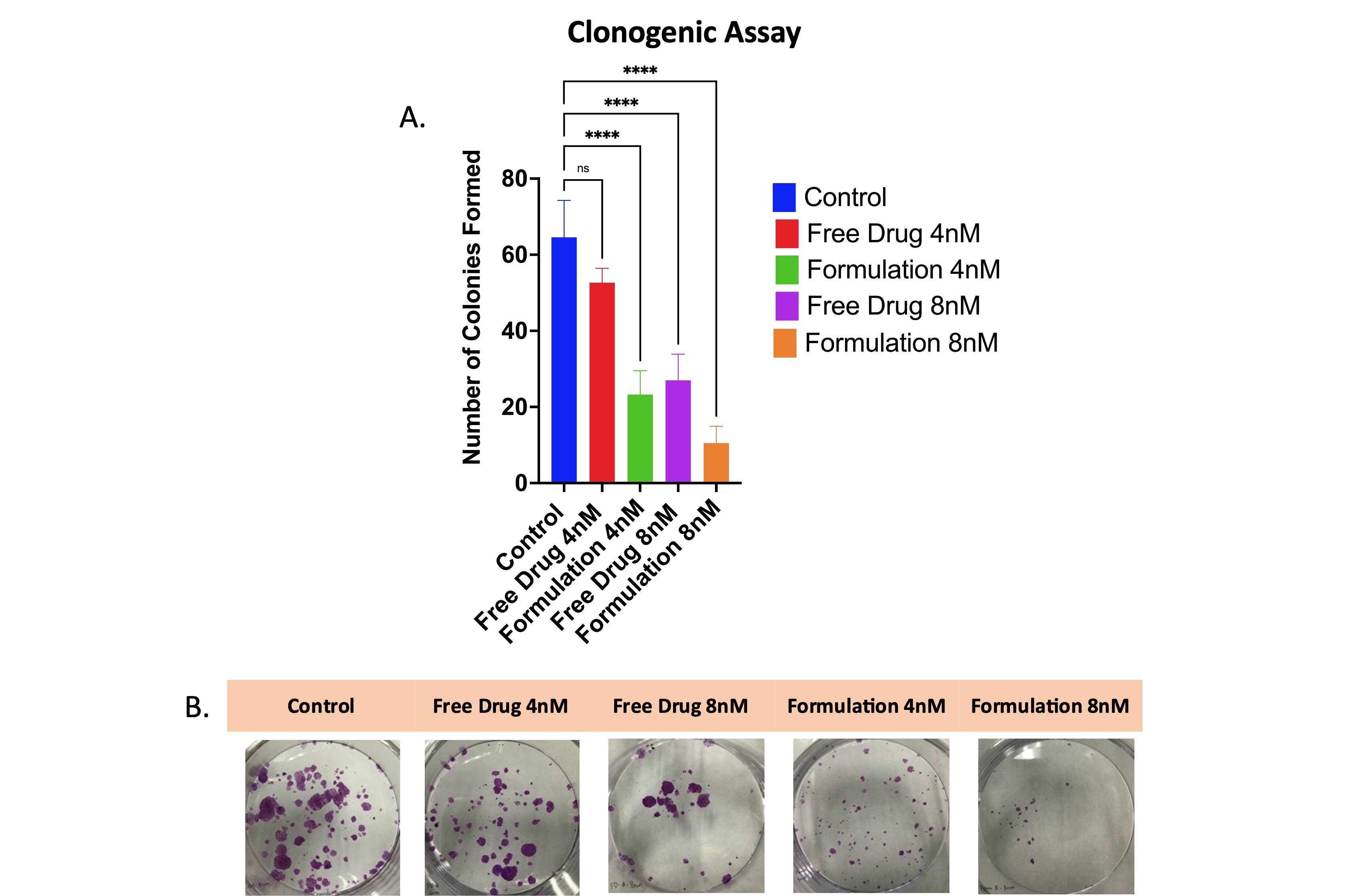 Figure 3: A) Clonogenic assay analysis after 48 h of GEF and GEFSLNs treatment at 4 nM and 8 nM. B) Images of colonies stained with crystal violet post 10 days of incubation in fresh media (n = 4, Mean ± SD). **** p < 0.0001.
Figure 3: A) Clonogenic assay analysis after 48 h of GEF and GEFSLNs treatment at 4 nM and 8 nM. B) Images of colonies stained with crystal violet post 10 days of incubation in fresh media (n = 4, Mean ± SD). **** p < 0.0001.