Formulation and Delivery - Chemical
Category: Poster Abstract
(M0930-11-73) Formulation Optimization of EVX-101, a Novel Gastro-Retentive Prolonged-Release 5-HTP/Low-Dose Carbidopa Tablet
- AR
Aruna Railkar, Ph.D.
Quotient Sciences
Nottingham, England, United Kingdom - VZ
Vanessa Zann, Ph.D.
Quotient Sciences
Nottingham, England, United Kingdom - BB
Bret Berner, Ph.D.
Pharmaceutical Consulting
Seattle, Washington, United States - DC
David Carpenter, Pharm.D.
Evecxia Therapeutics, Inc.
Durham, North Carolina, United States - JR
Joel Raskin, M.D.
Evecxia Therapeutics, Inc.
Durham, North Carolina, United States - JJ
Jacob Jacobsen, Ph.D.
Evecxia Therapeutics, Inc
North Carolina, North Carolina, United States - LM
Litza McKenzie, M.D.
Quotient Sciences
Nottingham, England, United Kingdom - BB
Beth Brickhill, BS
Quotient Sciences
Brisbane, England, United Kingdom - SB
Sarah Beaudoin
Quotient Sciences
Brisbane, England, United Kingdom - CT
Clare Totaro
Quotient Sciences
Nottingham, England, United Kingdom 
Ching Sieu Tay, MS (she/her/hers)
Director of Development
Quotient Sciences
Nottingham, England, United Kingdom- WL
Wu Lin, Ph.D. (he/him/his)
Quotient Sciences
Nottingham, England, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Inadequate response to antidepressant treatment is a significant issue for many patients with depression. Enhancing extracellular serotonin (5-HT) levels beyond those produced by serotonin-reuptake inhibitors may treat depression more effectively. Adjunctive treatment with 5-hydroxytryptophan (5-HTP), the natural precursor to 5-HT, can amplify brain serotonin. However, 5-HTP absorption is restricted to the upper intestine, bioavailability is low, and 5-HTP has a short half-life (t½), making 5-HTP a poor drug. To overcome these pharmacokinetic (PK) limitations, a gastro-retentive (GR) sustained-release (SR) formulation is required to maximize absorption producing sustained exposure. Carbidopa inhibits amino acid decarboxylase, the enzyme that converts 5-HTP to 5-HT in the intestine, resulting in decreased first pass metabolism and increased 5-HTP bioavailability. The development and optimization of EVX-101, a novel twice a day GR SR tablet 5-HTP/carbidopa formulation targeting steady-state average 5-HTP plasma concentration (ssCavg) of 150–250 ng/mL is described.
Methods: A two-part clinical study was conducted in healthy volunteers using Translational Pharmaceutics® (rapid manufacturing and clinical testing), allowing application of flexible formulation design space to optimize the formulation based on emerging clinical data. Part 1 assessed proof of concept (POC) for a GR formulation, using a sipping protocol design to simulate GR input rate by administering doses in 10 equal aliquots every hour over 9 hours, and assessed the impact of low dose carbidopa (0 – 10 mg) on the bioavailability of 5-HTP (250 mg). Twelve healthy subjects were dosed in five sequential periods, assessing 5-HTP exposure in response to various carbidopa doses. Part 1 data was used to define the carbidopa dose range for the GR tablets for Part 2. The tablet contained a GR layer and a modified-release (MR) layer containing a fixed 5-HTP dose (250 mg) and a two-dimensional design space (Figure 1) for variable carbidopa dose (0.3125–25 mg) and variable hypromelloses to control release rate of 5-HTP and carbidopa (target 80% from 8.5 to 12 hours). The tablet was radiolabeled with 111Indium so scintigraphic imaging could be used to assess in-vivo tablet performance. Part 2 dosed a new cohort of 16 subjects in five sequential periods, to assess GR tablet performance dosed in the fed state (high fat breakfast), based on gastric emptying (GE) time, gastrointestinal (GI) transit parameters, and tablet disintegration. The carbidopa dose-response relationship to 5-HTP exposure and the impact of dosing with a moderate fat meal were also assessed. For both parts, interim decisions occurred between dosing periods, whereby PK, safety, and scintigraphy data (Part 2 only) were reviewed to assess formulation performance and decide upon the carbidopa dose and formulation composition for the next dosing period.
Results: In Part 1, carbidopa had the anticipated effect on 5-HTP exposure, showing increased exposure and t½, and confirming POC for a GR formulation. The initial GR prototype assessed in Part 2 was 250 mg 5-HTP and 0.625 mg carbidopa with a 5-HTP target release rate of 80% at 8.5 hours. Scintigraphic images (Figure 2) showed a mean GE time of 5.9 hours. As appropriate GR was achieved with prototype 1, the 5-HTP release rate was fixed and subsequent dosing periods were used to explore the carbidopa dose response (2.5, 5, and 15 mg) and assess the impact of a moderate fat meal. Increasing the carbidopa dose resulted in further increases in 5-HTP exposure (Figure 3). Dosing with a moderate fat meal resulted in faster GE with a mean of 4.4 hours, as expected with a reduction in meal fat content. Intestinal transit parameters, including GE, were acceptable for a viable GR formulation for both high- and moderate-fat breakfasts. Modeling showed that the target ssCavg could be achieved by several of the formulation prototypes dosed in Part 2. Review of safety data (adverse events [AEs], clinical laboratory tests, electrocardiograms, and vital signs) showed a safety profile consistent with the known safety profile of 5-HTP. The most frequently reported AEs were GI in nature (nausea and vomiting), followed by neurological (headache), and were mostly mild to moderate. Doses that achieved the target ssCavg were safe and tolerated by healthy volunteers.
Conclusion: Translational Pharmaceutics allowed emerging clinical data to be used within a clinical study to validate POC for a GR formulation, refine the carbidopa dose range, confirm MR release rate and GR properties, and identify an EVX-101 GR tablet to achieve the target profile. The optimized EVX-101 formulation was recently used to successfully complete a single and multiple ascending dose study.
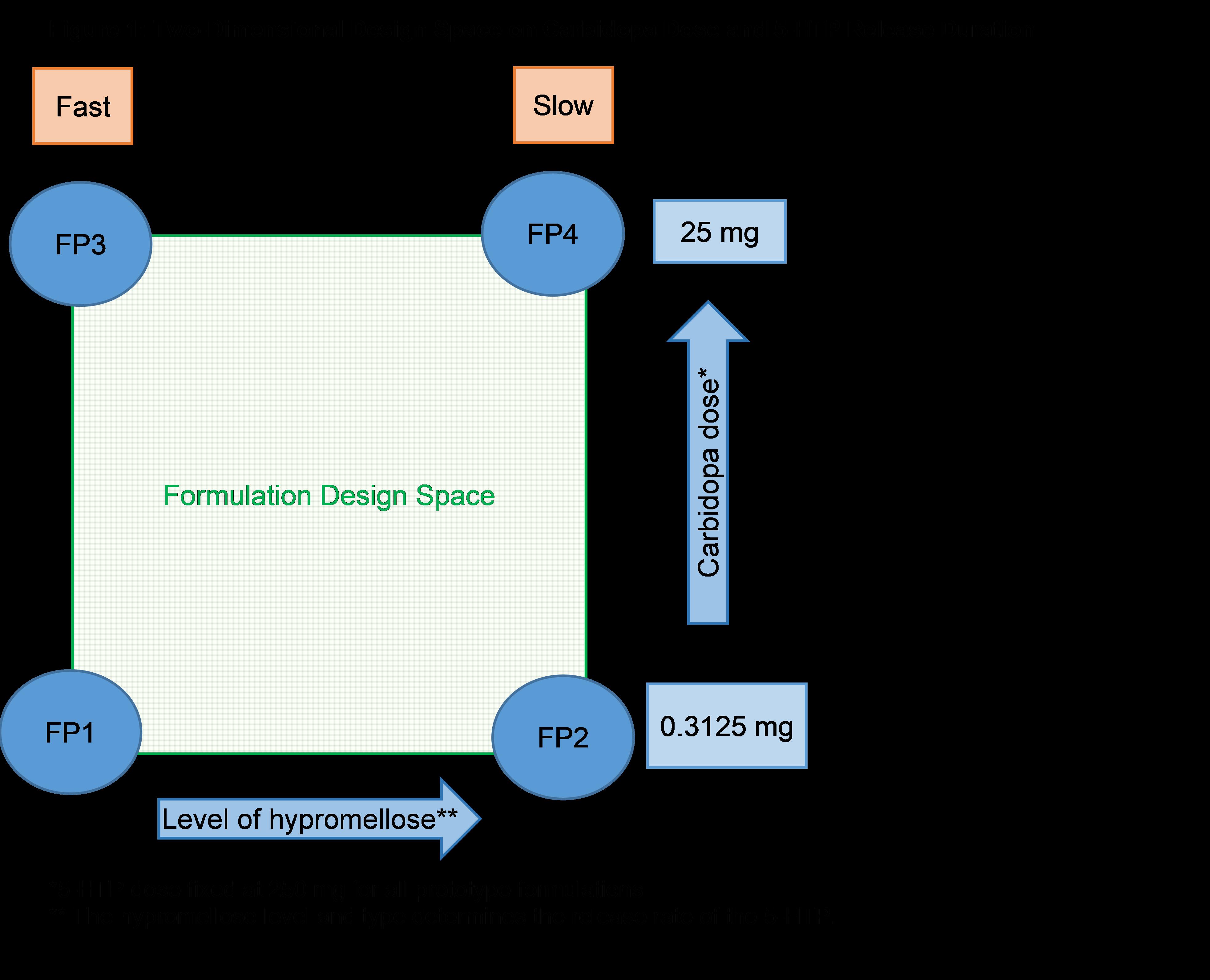 Figure 1: Two-dimensional design space for the MR layer
Figure 1: Two-dimensional design space for the MR layer 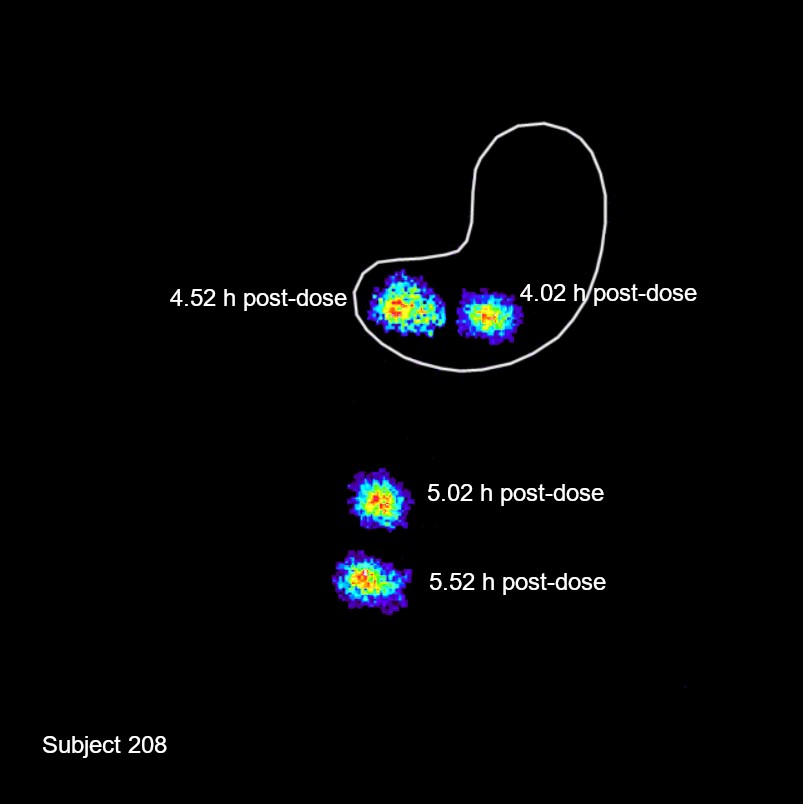 Figure 2: Scintigraphic images showing GE
Figure 2: Scintigraphic images showing GE 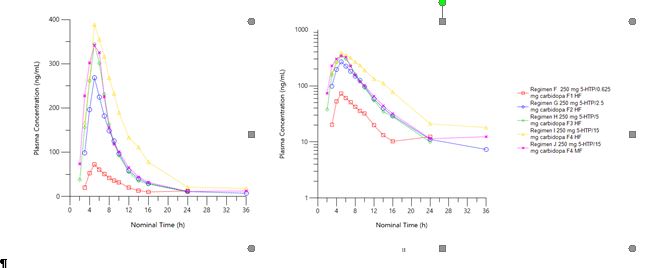 Figure 3. Mean plasma concentration-time profiles for 5-HTP when dosed with various EVX-101 GR prototype formulations (250 mg 5-HTP and 0.625–15 mg carbidopa), HF = high fat, MF = moderate fat
Figure 3. Mean plasma concentration-time profiles for 5-HTP when dosed with various EVX-101 GR prototype formulations (250 mg 5-HTP and 0.625–15 mg carbidopa), HF = high fat, MF = moderate fat